- Home
- Resource
- Explore & Learn
- Artificial Antibodies: The Future of Diagnostics and Therapeutics
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
In the rapidly evolving field of in vitro diagnostics (IVD) and therapeutics, the quest for reliable, cost-effective, and stable alternatives to natural antibodies has intensified. Natural antibodies, while indispensable in modern medicine, are fraught with challenges including high production costs, stringent storage requirements, and limited stability. These limitations have spurred the development of Molecularly Imprinted Polymers (MIPs)—synthetic materials engineered to mimic the binding specificity and affinity of natural antibodies. Often referred to as "artificial antibodies" or "plastic antibodies," MIPs promise to revolutionize diagnostics and therapeutics by offering scalable, robust, and economically viable solutions.
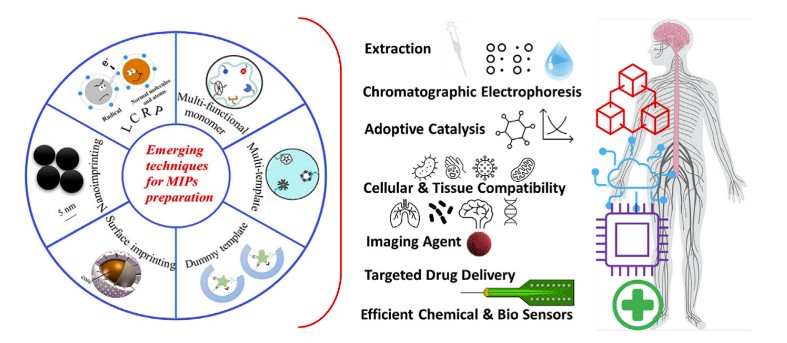 Fig.1 Illustration of MIP development according to targeted biological templates and potential application of high-performance MIPs for advanced biomedical applications. (Dixit C. K., et al., 2022)
Fig.1 Illustration of MIP development according to targeted biological templates and potential application of high-performance MIPs for advanced biomedical applications. (Dixit C. K., et al., 2022)
Molecular imprinting is a sophisticated technique that involves creating template-shaped cavities within a polymer matrix. These cavities, or imprints, are designed to recognize and bind specific target molecules with high affinity and specificity. The process typically involves three key steps:
MIPs offer several distinct advantages over natural antibodies:

MIP-based biosensors have emerged as a powerful tool for rapid, sensitive, and specific detection of biomarkers. For instance, researchers have developed MIP-based electrochemical sensors for detecting cancer biomarkers, such as the gaseous non-anal cancer biomarker at a limit of detection (LOD) of 4.5 ppm. Similarly, MIP-based optical sensors using quartz crystal microbalance (QCM) and surface plasmon resonance (SPR) have demonstrated high sensitivity and selectivity for detecting environmental contaminants like 2,4-dichlorophenoxyacetic acid (2,4-D) in apple samples.

MIPs are increasingly being integrated into immunoassays and lateral flow devices (LFDs) as replacements for natural antibodies. Magnetic MIPs, for example, can provide retrievable surfaces with antibody-like pockets, enabling wash-free single-step assays similar to PerkinElmer's AlphaLISA. This innovation significantly reduces assay time and reagent costs, making MIPs ideal for high-throughput screening in clinical settings.

The ability to imprint multiple templates on a single MIP support opens the door to multiplexed detection. This capability is particularly valuable in diagnostics, where simultaneous detection of multiple biomarkers can enhance disease diagnosis accuracy. For instance, MIP-based arrays have been developed for detecting various proteins, antibiotics, and even whole bacteria, demonstrating their potential in complex biological sample analysis.
Scalability and Manufacturing
One of the primary challenges hindering the widespread adoption of MIPs is the scalability of their production. While laboratory-scale synthesis of MIPs is well-established, translating these processes into industrial-scale manufacturing remains a hurdle. Issues such as polymer wastage, batch-to-batch variability, and the need for standardized protocols must be addressed to ensure consistent quality and performance of MIP-based products.
Specificity and Sensitivity
Achieving high specificity and sensitivity comparable to natural antibodies is crucial for MIPs to gain acceptance in diagnostics and therapeutics. Non-specific binding, template leakage, and mass transfer limitations are common issues that can compromise MIP performance. Ongoing research is focused on developing advanced polymerization techniques, such as reversible addition fragmentation chain transfer (RAFCT) and electropolymerization, to improve MIP specificity and sensitivity.
Integration with Emerging Technologies
The integration of MIPs with emerging technologies like artificial intelligence (AI), Internet of Things (IoT), and smart sensing platforms holds immense promise. AI-driven algorithms can optimize MIP design and synthesis, while IoT-enabled devices can facilitate real-time monitoring and data analysis. Smart sensing platforms, combining MIPs with nanomaterials and microfluidics, can enable high-throughput, multiplexed detection in point-of-care settings.
Artificial antibodies, in the form of MIPs, represent a groundbreaking advancement in the fields of diagnostics and therapeutics. Their stability, reusability, and cost-effectiveness make them attractive alternatives to natural antibodies, with the potential to democratize access to advanced medical technologies. While challenges remain, ongoing research and development efforts are steadily overcoming these hurdles.
The future of MIPs is bright, with academic-industry partnerships driving innovation and commercialization. As regulatory frameworks evolve to accommodate these novel materials, MIPs are poised to play a pivotal role in shaping the next generation of medical diagnostics and therapeutics. The journey from laboratory curiosity to mainstream clinical application may be challenging, but the rewards—a more accessible, efficient, and sustainable healthcare system—are well worth the effort.
If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| AADA-HMM-0004 | Dextromethorphan (DXM)-02 | Add To Cart |
| AAKIM-HMM-0002 | Urinary Microalbumin (HSA[MAU])-04 | Add To Cart |
| AADA-HMM-0006 | Dextromethorphan (DXM)-40 | Add To Cart |
| AACB-HMM-0012 | Procalcitonin (PCT)-01 | Add To Cart |
| AACB-HMM-0004 | Heart-type Fatty Acid-bindin Protein (H-FABP)-01 | Add To Cart |
| AAID-HMM-0007 | Influenza A Virus (FluA)-09 | Add To Cart |
| AACB-HMM-0015 | Procalcitonin (PCT)-09 | Add To Cart |
| AALIM-HMM-0003 | Glycocholic Acid (CG) -98 | Add To Cart |
| AADA-HMM-0003 | Etomidate (ETO) | Add To Cart |
| AACB-HMM-0009 | D-Dimer-06 | Add To Cart |
| AACB-HMM-0010 | D-Dimer-10 | Add To Cart |
| AACB-HMM-0002 | N-terminal pro-brain natriuretic peptide (NT-ProBNP)-02 | Add To Cart |
| AATM-HMM-0005 | Carbohydrate Antigen 125 (CA125)-01 | Add To Cart |
| CLRM-HMM-0002 | DNP-BSA | Add To Cart |
| AATM-HMM-0003 | Ferritin-03 | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |