- Home
- Resource
- Explore & Learn
- Aptamer-Based Lateral Flow Assay (ALFA) Without Biotin/Avidin for Trichomonas vaginalis Diagnosis
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Trichomonas vaginalis, an anaerobic flagellated parasite, is the causative agent of trichomoniasis, the most prevalent non-viral sexually transmitted infection (STI). The World Health Organization (WHO) estimates 156.3 million new cases annually among adults aged 15–49, with transmission often leading to severe health consequences, including increased risk of HIV acquisition, preterm birth, and cervical dysplasia. Traditional diagnostic methods—such as wet-mount microscopy, culture, and nucleic acid amplification tests (NAATs)—face critical limitations: wet-mount lacks sensitivity (50–60%), culture requires specialized labs, and NAATs are costly and equipment-dependent. This gap underscores the urgent need for a rapid, affordable, and accessible point-of-care test (POCT) aligned with WHO's REASSURED criteria.
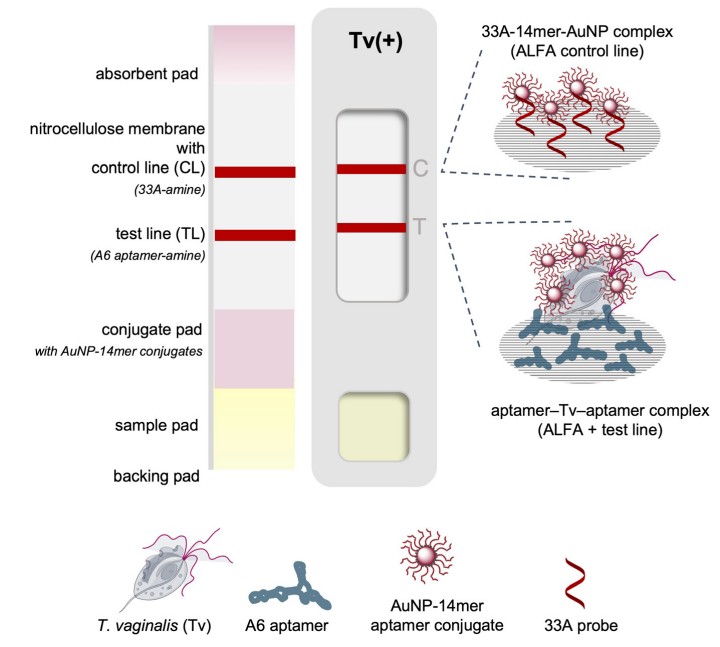 Fig.1 Schematic of the biotin/neutravidin-free ALFA for T. vaginalis. (Justo C. A. C., et al., 2025)
Fig.1 Schematic of the biotin/neutravidin-free ALFA for T. vaginalis. (Justo C. A. C., et al., 2025)
Commercially available POCTs for T. vaginalis remain limited. The OSOM Trichomonas Rapid Test, an immunochromatographic assay, offers 10-minute results but relies on antibody-based detection with a limit of detection (LOD) of 2.5 × 103 cells/mL. The Visby Medical Sexual Health Test integrates automated PCR for multi-pathogen detection but is designed exclusively for vaginal swabs and requires power sources. Both assays fail to address key needs: male sample compatibility, low-cost production, and long-term stability. As of 2025, only two CLIA-waived tests exist, highlighting a critical market gap for robust, user-friendly diagnostics.
Mechanism of Action
The ALFA leverages DNA aptamers—synthetic oligonucleotides with high target affinity—to enable a biotin/avidin-free sandwich assay. The design employs two aptamers: A6 (95-mer) as a capture probe immobilized on a nitrocellulose membrane via UV cross-linking, and A1_14mer (14-mer) functionalized with thioctic acid-conjugated gold nanoparticles (AuNPs) as a reporter. When a sample containing T. vaginalis is applied, the A1_14mer-AuNP conjugate binds to the parasite, which is then captured by A6 at the test line, forming a visible red band. A control line with polyA oligonucleotide (33A) ensures assay validity.

The ALFA detects as low as 1.6×105 T. vaginalis cells/mL, outperforming antibody-based LFAs for Neisseria gonorrhoeae (106 cells/mL) but slightly lower than its microplate aptamer assay counterpart (3.02×103 cells/mL). Serial dilution studies confirmed linearity across 2.0×107 to 1.6×105 cells/mL, with signal intensity measured via the Cube LFA reader.
Specificity testing against common vaginal microbes—Candida albicans, Gardnerella vaginalis, Klebsiella pneumoniae, and multiple Lactobacillus species—showed no cross-reactivity, even at high concentrations (e.g., 1.5×108 cells/mL for bacteria). Test line intensities remained near zero for non-target organisms, confirming high selectivity.

The ALFA strip and running buffer demonstrate exceptional stability: stored at 4°C or 37°C for 90 days, the assay retained functionality, with Arrhenius modeling predicting a 1-year shelf-life at 22°C. This stability surpasses many commercial POCTs, which often require refrigeration.
Production costs are remarkably low, totaling < 1€ per test. Key cost drivers include inexpensive materials (nitrocellulose membrane, glass fiber pads) and aptamer synthesis, with the AuNP-aptamer conjugate accounting for ~15% of the total cost.
Testing with four residual CVL samples from the AVEONS study showed 100% concordance with wet-mount microscopy. Notable findings included:
The ALFA requires three user-friendly steps:
This workflow aligns with WHO's POCT target product profile, eliminating the need for trained personnel or equipment. Visual interpretation (two red lines for positive, one for negative) ensures accessibility in resource-limited settings.
Ongoing research aims to enhance ALFA performance:
The aptamer-based ALFA represents a paradigm shift in T. vaginalis diagnostics, combining high sensitivity (1.6×10⁵ cells/mL), specificity, and unprecedented affordability (< 1€/test). Its stability, user-friendly design, and clinical validation position it as a cornerstone for the WHO's 2030 goal of eliminating STIs. By addressing key limitations of existing POCTs, the ALFA holds tremendous potential to improve global access to timely trichomoniasis diagnosis, particularly in low- and middle-income countries, and mitigate the far-reaching health impacts of this neglected STI.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |