- Home
- Resource
- Explore & Learn
- Advances in Point-of-Care Malaria Diagnostics: A Comprehensive Scoping Review
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Malaria, a life-threatening disease caused by Plasmodium parasites transmitted via Anopheles mosquitoes, continues to pose a significant public health challenge. In 2022, the World Health Organization (WHO) reported 249 million clinical cases and 619,000 deaths, with sub-Saharan Africa accounting for 95% of cases and children under five years old constituting 80% of fatalities.
Accurate and timely diagnosis is the linchpin in malaria control. It ensures appropriate treatment, curbs the development of drug resistance, and facilitates disease surveillance. In regions with limited healthcare infrastructure, where access to centralized laboratories is scarce, point-of-care (POC) diagnostics have emerged as a crucial solution.
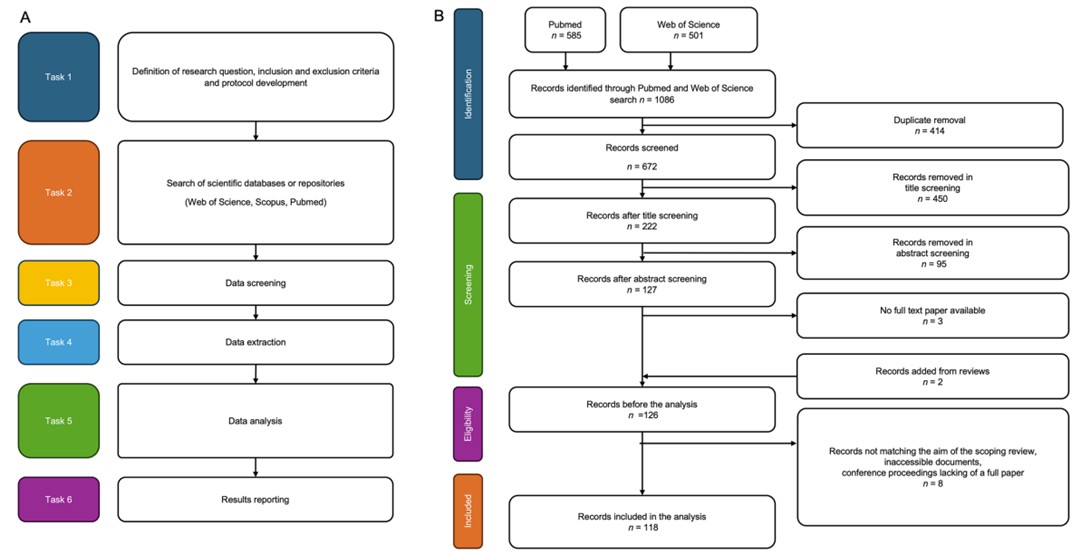 Fig.1 A Overview of the search strategy. B Flow diagram of the screening protocol according to PRISMA guidelines. (Coro F., et al., 2025)
Fig.1 A Overview of the search strategy. B Flow diagram of the screening protocol according to PRISMA guidelines. (Coro F., et al., 2025)
The WHO's ASSURED criteria—Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, and Deliverable—serve as a guiding principle for the development of POC malaria tests suitable for low-resource settings (LRS).
For widespread adoption in LRS, malaria POC tests should cost between $0.50 and $1 per test. High-cost components, such as specialized antibodies or complex manufacturing processes, often render tests unaffordable. For example, some advanced molecular tests that incorporate proprietary technologies can cost several times this amount, making them inaccessible to the populations most in need.
A highly sensitive test minimizes false negatives, ensuring that infected individuals receive timely treatment. Conversely, a specific test reduces false positives, preventing unnecessary medication and resource wastage. Rapid diagnostic tests (RDTs), the most commonly used POC malaria tests, have variable sensitivity and specificity. Some RDTs may miss low-level infections, leading to untreated cases that can contribute to disease spread.
POC tests must be easy to use by non-specialized personnel, provide results quickly (preferably within 30 minutes), function without complex equipment, and withstand harsh environmental conditions during storage and transportation. RDTs meet many of these criteria, but even they may require basic tools like lancets for blood collection and may be affected by extreme temperatures.
Immunoassays
Immunoassays, particularly RDTs, dominate the malaria POC diagnostic market. These tests detect specific Plasmodium antigens, such as histidine-rich protein II (HRP2) or Plasmodium lactate dehydrogenase (pLDH), in blood samples. RDTs are simple lateral-flow devices that produce visible lines indicating the presence of the target antigen.
However, they have limitations. In regions where Plasmodium falciparum has developed HRP2-deletion mutations, HRP2-based RDTs may yield false negatives. Additionally, most RDTs cannot differentiate between Plasmodium species, which is important for treatment selection as different species may respond differently to anti-malarial drugs.
Molecular Methods
Loop-Mediated Isothermal Amplification (LAMP)
LAMP is a nucleic acid amplification technique that operates at a constant temperature, eliminating the need for expensive thermal cyclers. It has high sensitivity, capable of detecting as few as 0.01 parasites per microliter of blood. Some LAMP-based POC tests can provide results in less than an hour and offer a visual readout through color changes.
For instance, the TwistDx Eiken RealAmp Malaria LAMP assay has been shown to accurately detect malaria parasites in field settings. However, many LAMP tests still require basic laboratory infrastructure, such as centrifuges for sample preparation, and are currently at a relatively low Technology Readiness Level (TRL), limiting their widespread use.

Polymerase Chain Reaction (PCR)
PCR is the gold standard for malaria diagnosis in terms of accuracy, as it can detect low-level infections and precisely identify Plasmodium species. However, it is not practical for LRS due to its requirement for specialized equipment, trained personnel, and a relatively long turnaround time.
Optical Microscopy
Optical microscopy, involving the examination of stained blood smears under a microscope, remains a widely used diagnostic method in malaria-endemic regions. It provides valuable information about parasite density, which is crucial for assessing disease severity and guiding treatment decisions.
However, microscopy requires well-trained microscopists, reliable microscopes, and a steady supply of staining reagents and electricity. In remote areas, these resources are often scarce, limiting their effectiveness as a POC diagnostic tool.
As mentioned, no single POC diagnostic technology fully meets all the ASSURED criteria. While RDTs are user-friendly and rapid, they lack sensitivity and specificity in some cases. Molecular methods like LAMP and PCR offer high accuracy but face challenges in terms of affordability, equipment-free operation, and ease of use in resource-constrained environments.
The majority of malaria POC tests rely on blood samples, which require invasive collection methods. This can be a barrier, especially in pediatric populations or in areas where people have a fear of needles. Non-invasive sample types, such as urine or saliva, have the potential to overcome these challenges but are under-explored, with only a small percentage of studies focusing on them.
Studies show that only 28% of research on malaria POC diagnostics includes authors from malaria-endemic countries. Local researchers are better equipped to understand the specific needs and challenges of their regions, such as extreme environmental conditions, limited healthcare infrastructure, and cultural factors that may affect test acceptance and use.
AI and machine learning algorithms are being applied to improve the accuracy and speed of malaria diagnosis. Automated image analysis systems can analyze blood smear images captured by portable microscopes, identifying malaria parasites with high precision.
For example, smartphone-based microscopy platforms, combined with AI-powered image recognition software, can provide accurate results in real-time, even in the hands of non-expert users. These systems can also be trained to detect specific Plasmodium species, adding valuable diagnostic information.
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology, known for its gene-editing capabilities, is also being developed for malaria diagnosis. CRISPR-based tests can detect specific Plasmodium DNA sequences with high sensitivity and specificity.
They can produce visual results, such as color changes, making them suitable for POC use. Although still in the early stages of development (TRL 3-4), CRISPR-based diagnostics have the potential to revolutionize malaria POC testing.
Innovations in manufacturing, such as 3D printing and open-source designs, are enabling the production of low-cost POC diagnostic devices. 3D-printed components can be used to create affordable microscopes or test cartridges, reducing production costs and allowing for local manufacturing in LRS. Open-source designs promote collaboration and adaptation of diagnostic technologies to local needs.
To overcome the existing challenges and improve malaria POC diagnostics, several key strategies should be pursued.
There is a need to focus on developing tests that meet all the ASSURED criteria. This includes further research on non-invasive sample collection methods, improving the affordability and user-friendliness of molecular tests, and enhancing the sensitivity and specificity of existing immunoassays.
Increasing the involvement of researchers and healthcare professionals from malaria-endemic countries in the development process is essential. This can be achieved through partnerships, training programs, and funding initiatives that support local research and innovation.
Once effective POC diagnostic technologies are developed, efforts must be made to scale up production and ensure widespread implementation. This requires strong supply chain management, training of healthcare workers, and education of the local population about the importance of malaria diagnosis and treatment.
In conclusion, while significant progress has been made in malaria POC diagnostics, there is still a long way to go. Through continued innovation, collaboration, and a focus on the specific needs of malaria-endemic regions, we can develop and implement effective POC diagnostic solutions that will play a crucial role in the global fight against malaria.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |