- Home
- Resource
- Explore & Learn
- A Humanized Mouse Model Featuring Markedly Enhanced Class-Switched, Antigen-Specific Antibody Responses
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Humanized mouse models have emerged as indispensable tools in immunological research, bridging the gap between mouse and human immune systems. These models involve the engraftment of human immune cells or tissues into immunodeficient mice, enabling the study of human immune responses in a controlled, in vivo environment. The quest for more physiologically relevant models has driven the development of sophisticated humanized mouse strains, with the incorporation of human cytokines playing a pivotal role.
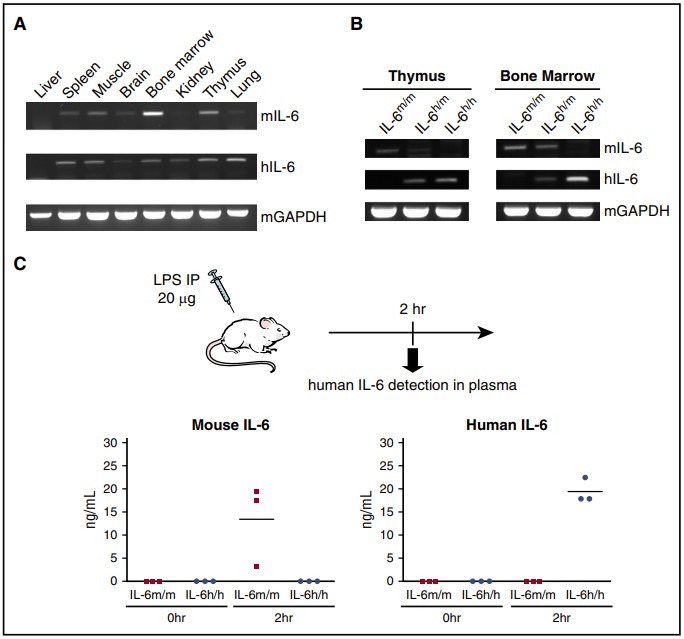 Fig.1 Validation of human IL-6 expression in knock-in mice. (Yu H., et al., 2017)
Fig.1 Validation of human IL-6 expression in knock-in mice. (Yu H., et al., 2017)
Cytokines are small proteins that play crucial roles in regulating immune responses. They act as messengers between cells, orchestrating the complex interplay of immune cells during inflammation, infection, and tissue repair. Among these, interleukin-6 (IL-6) stands out for its multifaceted role in both innate and adaptive immunity. IL-6 is involved in B-cell differentiation, T-cell activation, and the acute-phase response, making it a key player in the immune system's ability to combat pathogens and maintain homeostasis.
Despite their utility, conventional humanized mouse models have limitations, particularly in generating robust, antigen-specific antibody responses. One major challenge has been the inefficient class-switching from IgM to IgG antibodies, a process critical for the development of high-affinity, long-lasting immunity. This limitation stems, in part, from the lack of adequate human cytokine support, particularly IL-6, which is essential for B-cell maturation and antibody production.

To overcome these limitations, researchers have developed human IL-6 knock-in mouse models. These models involve the insertion of the human IL-6 gene into the mouse genome, replacing the mouse IL-6 gene. This genetic modification ensures the physiological expression of human IL-6, which is crucial for the proper differentiation and function of human immune cells engrafted in the mice.

Studies have demonstrated that human IL-6 knock-in mice generate robust antigen-specific antibody responses. Upon immunization with ovalbumin (OVA), these mice produce high levels of OVA-specific IgG antibodies. This is in stark contrast to conventional humanized mouse models, which often fail to produce significant amounts of antigen-specific IgG.
The antibodies produced in human IL-6 knock-in mice exhibit a diverse repertoire and high frequency of somatic hypermutation. This indicates efficient B-cell activation and selection, processes that are critical for the generation of high-affinity antibodies. The presence of somatic hypermutation suggests that human IL-6 supports the germinal center reaction, where B cells undergo affinity maturation.

Interestingly, a subset of antibodies cloned from IgG-switched B cells in human IL-6 knock-in mice displayed polyreactivity, binding to multiple antigens with low affinity. This feature, reminiscent of antibodies found in healthy donors, may confer broad antibacterial activity. The ability to produce polyreactive antibodies highlights the versatility of the human IL-6 knock-in mouse model in mimicking human immune responses.
If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| AADA-HMM-0004 | Dextromethorphan (DXM)-02 | Add To Cart |
| AAKIM-HMM-0002 | Urinary Microalbumin (HSA[MAU])-04 | Add To Cart |
| AADA-HMM-0006 | Dextromethorphan (DXM)-40 | Add To Cart |
| AACB-HMM-0012 | Procalcitonin (PCT)-01 | Add To Cart |
| AACB-HMM-0004 | Heart-type Fatty Acid-bindin Protein (H-FABP)-01 | Add To Cart |
| AAID-HMM-0007 | Influenza A Virus (FluA)-09 | Add To Cart |
| AACB-HMM-0015 | Procalcitonin (PCT)-09 | Add To Cart |
| AALIM-HMM-0003 | Glycocholic Acid (CG) -98 | Add To Cart |
| AADA-HMM-0003 | Etomidate (ETO) | Add To Cart |
| AACB-HMM-0009 | D-Dimer-06 | Add To Cart |
| AACB-HMM-0010 | D-Dimer-10 | Add To Cart |
| AACB-HMM-0002 | N-terminal pro-brain natriuretic peptide (NT-ProBNP)-02 | Add To Cart |
| AATM-HMM-0005 | Carbohydrate Antigen 125 (CA125)-01 | Add To Cart |
| CLRM-HMM-0002 | DNP-BSA | Add To Cart |
| AATM-HMM-0003 | Ferritin-03 | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |