- Home
- Resource
- Explore & Learn
- A Comprehensive Guide to Toxicology in Clinical Laboratories
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Toxicology, the study of the adverse effects of chemicals on living organisms, plays a pivotal role in clinical laboratories. With the rising prevalence of drug abuse and the introduction of novel psychoactive substances (NPS), the demand for accurate and timely toxicological analyses has never been greater. This comprehensive guide delves into the intricacies of toxicology in clinical settings, highlighting recent advancements, challenges, and future directions.
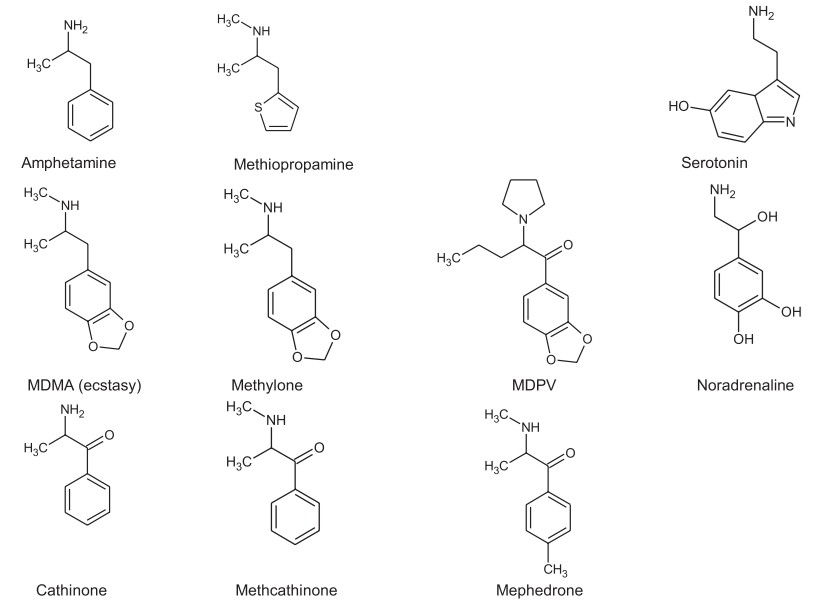 Fig.1 Structures of amphetamine, ecstasy and cathinone and a number of derivatives, with the structures of serotonin and noradrenaline for comparison. (Brown N. W., et al., 2017)
Fig.1 Structures of amphetamine, ecstasy and cathinone and a number of derivatives, with the structures of serotonin and noradrenaline for comparison. (Brown N. W., et al., 2017)
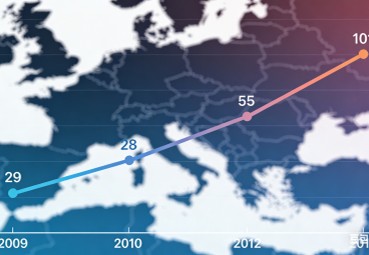
The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) has documented a dramatic surge in the prevalence of novel psychoactive substances (NPS) across Europe. In just a few years, the number of seized NPS skyrocketed from 29 in 2009 to a peak of 101 in 2014. These substances, often deceptively marketed as "legal highs," have introduced a new layer of complexity for toxicologists. Their diverse chemical structures, rapid turnover, and the limited availability of reference standards pose significant challenges for accurate detection and identification. The ever-evolving nature of NPS means that toxicologists must constantly adapt to new compounds and formulations, making it difficult to keep pace with the illicit market.

Detecting NPS in clinical samples is further complicated by their extensive metabolism. Many of these substances undergo significant biotransformation, leading to low or undetectable levels of the parent compound in urine. This metabolic complexity means that traditional screening methods, such as immunoassays, are often insufficient for detecting NPS. Instead, advanced mass spectrometric techniques, like liquid chromatography-tandem mass spectrometry (LC-MS/MS) and high-resolution mass spectrometry (HRMS), are increasingly relied upon to identify these substances.
For example, synthetic cannabinoids (SC), a class of NPS that bind to cannabinoid receptors with high affinity, are frequently detected in products marketed as "herbal" or "spice" blends. These compounds undergo complex metabolism, producing numerous metabolites that may be more detectable than the parent drug. However, the lack of standardized reference materials and the constant emergence of new SC variants make identification a daunting task. Each new variant may have a slightly different chemical structure, requiring updated detection methods and reference standards. This rapid evolution of NPS underscores the need for flexible and adaptable analytical techniques that can keep up with the changing landscape of drug use.

Rapid Screening with Immunoassays
Immunoassays, particularly automated enzyme-linked immunosorbent assays (ELISAs), are widely used in clinical laboratories for rapid drug screening. These assays offer high throughput, ease of use, and relatively low cost, making them ideal for initial screening in various healthcare settings. Many instant point-of-care tests (POCTs) also utilize immunoassay technology, enabling on-site drug testing in emergency departments, primary care clinics, and even in the field. The ability to provide quick results allows healthcare providers to make timely decisions regarding patient care, ensuring that individuals receive appropriate interventions as soon as possible.
False Positives and Negatives
Despite their widespread use and numerous advantages, immunoassays are prone to false positives and negatives, which can significantly impact the accuracy of drug screening results. Cross-reactivity with structurally similar compounds is a common issue that can lead to false positive results. For example, certain medications and dietary supplements have been reported to cause false positives in amphetamine and benzodiazepine immunoassays. Conversely, low drug concentrations or the presence of masking agents may result in false negatives, potentially overlooking substance use when it is present. These inaccuracies highlight the importance of using confirmatory testing methods, such as mass spectrometry, to validate initial screening results and ensure the reliability of drug testing outcomes.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |