- Home
- Resource
- Explore & Learn
- A Breakthrough in Rapid Liver Injury Detection: The SERS-LFIA Diagnostic
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Surface Enhanced Raman Scattering Lateral Flow Immunoassay (SERS-LFIA) represents a transformative innovation in the field of in vitro diagnostics, particularly for the rapid detection of drug-induced liver injury (DILI). Traditional diagnostic methods for liver injury, such as alanine aminotransferase (ALT) assays, often fail to provide timely and accurate results, especially within the critical therapeutic window for effective intervention. The SERS-LFIA diagnostic overcomes these limitations by offering a highly sensitive and specific point-of-care (POC) solution that can detect biomarkers of liver injury in under 30 minutes.
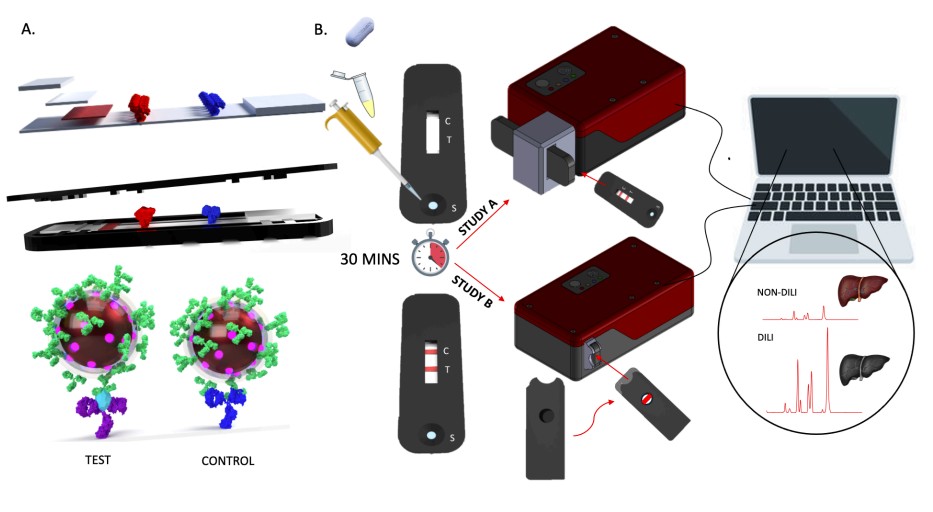 Fig.1 Pre-clinical SERS-LFIA development. (Dear J., et al., 2025)
Fig.1 Pre-clinical SERS-LFIA development. (Dear J., et al., 2025)
SERS-LFIA leverages the principles of Surface Enhanced Raman Scattering (SERS), a powerful vibrational spectroscopy technique that enhances the Raman scattering of molecules bound to a roughened metal surface. This enhancement is achieved by exciting surface plasmons, typically using gold or silver nanoparticles. In the context of SERS-LFIA, gold nanoparticles are functionalized with a Raman reporter molecule and antibodies specific to the target biomarker, such as cytokeratin-18 (K18). When a patient's serum sample is applied to the lateral flow strip, these nanoparticles bind to the biomarker, forming a sandwich immunoassay. The resulting SERS signal is then quantitatively measured using a handheld Raman reader (HRR), providing a precise measurement of the biomarker concentration.
The development of the SERS-LFIA diagnostic involves several critical steps, including nanoparticle synthesis, functionalization, and strip assembly. Gold nanoparticles are synthesized using a citrate reduction method and coated with a Raman reporter molecule, such as 4,4'-dipyridyl (DIPY), and encapsulated in a silica shell. Antibodies specific to K18 are then attached to the nanoparticles, creating a highly specific detection system. The lateral flow strip is assembled with treated sample pads, conjugate pads, test and control lines, and a collection pad, all housed in a 3D-printed cassette for ease of use.
Calibration of the SERS-LFIA device is performed using healthy human serum spiked with known concentrations of K18. The calibration curve demonstrates a strong linear relationship between the SERS signal and K18 concentration, with an R2 value of 0.98, indicating high accuracy and reproducibility. This calibration allows for the quantitative measurement of K18 in patient samples, facilitating early detection of liver injury.
The clinical validation of the SERS-LFIA diagnostic was conducted in two studies, involving a total of 199 serum samples from patients with paracetamol overdose. The studies evaluated the device's sensitivity, specificity, and overall diagnostic accuracy in detecting DILI.
Study A: Initial Evaluation
In Study A, 99 serum samples were analyzed in triplicate by three independent operators. The results showed a median K18 concentration of 161.0 ng/mL in DILI patients compared to 41.0 ng/mL in non-DILI patients. The diagnostic accuracy of the SERS-LFIA device was 87.9%, with a sensitivity of 81.6% and a specificity of 94.0%. The receiver operating characteristic (ROC) curve analysis yielded an area under the curve (AUC) of 0.93, indicating excellent diagnostic performance.
Study B: Refined Device Evaluation
Following refinements to the device based on feedback from Study A, Study B evaluated the SERS-LFIA in 100 serum samples. The refined device achieved a sensitivity of 82.0% and a specificity of 94.0%, with a median K18 concentration of 213.2 ng/mL in DILI patients compared to 55.6 ng/mL in non-DILI patients. The ROC-AUC for Study B was 0.97, demonstrating further improvements in diagnostic accuracy.
Traditional methods for detecting liver injury, such as ALT assays, often take several hours to produce results, delaying critical treatment decisions. In contrast, the SERS-LFIA diagnostic provides a rapid and quantitative result in under 30 minutes. The SERS-LFIA also offers higher sensitivity and specificity compared to traditional methods, as demonstrated in the clinical studies. For example, the SERS-LFIA achieved a sensitivity of 82.0% and a specificity of 94.0% in Study B, compared to the typical sensitivity of 57% for commercial LFIA tests.
The SERS-LFIA diagnostic holds significant potential for future applications beyond the detection of DILI. Its platform technology can be adapted to measure other biomarkers associated with various diseases, making it a versatile tool for in vitro diagnostics. Future developments may include the use of multiplexed assays to detect multiple biomarkers simultaneously, enhancing the diagnostic capabilities of the device.
Future research should focus on validating the SERS-LFIA diagnostic in larger and more diverse patient populations. Prospective clinical trials can further assess the device's performance in real-world settings, providing valuable data on its clinical utility. Additionally, studies should explore the device's potential for monitoring patients' responses to treatment and predicting outcomes in clinical trials of new therapies.
The portability and ease of use of the SERS-LFIA device make it suitable for point-of-care testing in various clinical settings, including emergency departments, rural clinics, and low-resource environments. Future development should aim to optimize the device for capillary blood testing, further expanding its applicability in point-of-care scenarios.
The SERS-LFIA diagnostic represents a significant advancement in the rapid detection of drug-induced liver injury. By combining lateral flow immunoassay with surface-enhanced Raman scattering, this innovative device provides a highly sensitive and specific point-of-care solution for identifying patients at risk of liver injury. With its rapid turnaround time and quantitative measurement capabilities, the SERS-LFIA diagnostic has the potential to revolutionize emergency care and improve patient outcomes. As this technology continues to evolve, it holds promise for transforming the field of in vitro diagnostics and addressing critical unmet needs in healthcare.
If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.

Cat.No. GP-DQL-00203
Rotavirus Antigen Group A and Adenovirus Antigen Rapid Test Kit (Colloidal Gold)

Cat.No. GP-DQL-00206
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Card Style

Cat.No. GP-DQL-00207
Adenovirus Antigen Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GP-DQL-00211
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. GP-DQL-00212
Rotavirus Antigen Group A Rapid Test Kit (Colloidal Gold), Card Type

Cat.No. IP-00189
Influenza A Rapid Assay Kit

Cat.No. GH-DQL-00200
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00201
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00202
Luteinizing Hormone Rapid Test Kit (Colloidal Gold)

Cat.No. GH-DQL-00208
Follicle-stimulating Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. GH-DQL-00209
Insulin-like Growth Factor Binding Protein 1 Rapid Test Kit(Colloidal Gold), Strip Style

Cat.No. GH-DQL-00210
Luteinizing Hormone Rapid Test Kit (Colloidal Gold), Strip Style

Cat.No. IH-HYW-0001
hCG Pregnancy Test Strip

Cat.No. IH-HYW-0002
hCG Pregnancy Test Cassette

Cat.No. IH-HYW-0003
hCG Pregnancy Test Midstream

Cat.No. GD-QCY-0001
Cocaine (COC) Rapid Test Kit

Cat.No. GD-QCY-0002
Marijuana (THC) Rapid Test Kit

Cat.No. GD-QCY-0003
Morphine (MOP300) Rapid Test Kit

Cat.No. GD-QCY-0004
Methamphetamine (MET) Rapid Test Kit

Cat.No. GD-QCY-0005
Methylenedioxymethamphetamine ecstasy (MDMA) Rapid Test Kit

Cat.No. GD-QCY-0006
Amphetamine (AMP) Rapid Test Kit

Cat.No. GD-QCY-0007
Barbiturates (BAR) Rapid Test Kit

Cat.No. GD-QCY-0008
Benzodiazepines (BZO) Rapid Test Kit

Cat.No. GD-QCY-0009
Methadone (MTD) Rapid Test Kit

Cat.No. GD-QCY-0011
Opiate (OPI) Rapid Test Kit

Cat.No. ID-HYW-0002
Multi-Drug Test L-Cup, (5-16 Para)

Cat.No. ID-HYW-0005
Multi-Drug Rapid Test (Dipcard & Cup) with Fentanyl

Cat.No. ID-HYW-0006
Multi-Drug Rapid Test (Dipcard & Cup) without Fentanyl

Cat.No. ID-HYW-0007
Multi-Drug 2~14 Drugs Rapid Test (Dipstick & Dipcard & Cup)

Cat.No. ID-HYW-0008
Fentanyl (FYL) Rapid Test (For Prescription Use)

Cat.No. ID-HYW-0009
Fentanyl Urine Test Cassette (CLIA Waived)

Cat.No. ID-HYW-0010
Fentanyl Urine Test Cassette (Home Use)
|
There is no product in your cart. |