X-Linked Agammaglobulinemia (XLA) is a rare but critical primary immunodeficiency caused by mutations in the BTK gene, leading to profound B-cell deficiency and recurrent life-threatening infections. Early and accurate diagnosis is essential to initiate life-saving treatments like immunoglobulin replacement therapy. This resource provides a comprehensive, standardized diagnostic workflow, from identifying clinical red flags to performing immunological testing and confirming genetic mutations, designed to help clinicians and laboratories overcome diagnostic challenges.
Introduction to X-Linked Agammaglobulinemia (XLA)
X-linked agammaglobulinemia (XLA) is a rare primary immunodeficiency caused by mutations in the BTK gene, leading to profound B-cell deficiency and severely reduced immunoglobulins (IgG, IgA, IgM). Characterized by recurrent bacterial infections (e.g., otitis media, pneumonia) typically emerging in infancy, XLA underscores the critical need for early diagnosis to prevent life-threatening complications. Diagnosis relies on a triad of clinical suspicion, immunological testing, and genetic confirmation of BTK mutations, enabling prompt initiation of life-saving immunoglobulin replacement therapy.
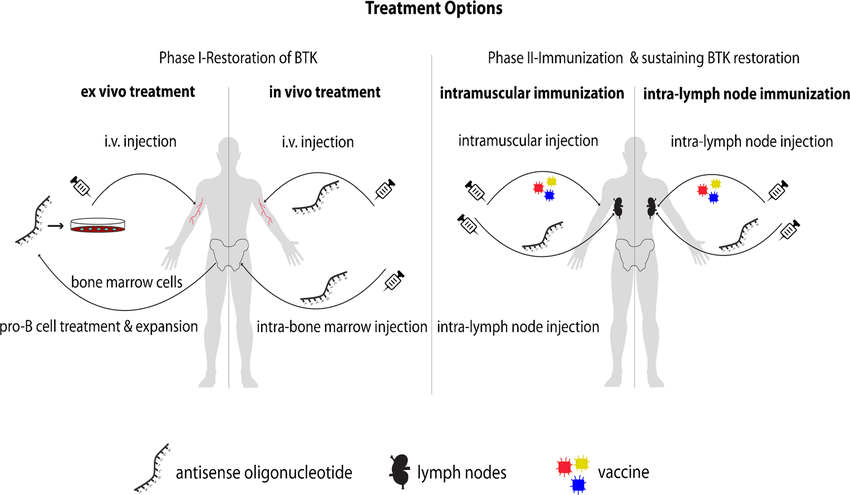 Fig.1 Proposed treatment options for X-linked agammaglobulinemia (XLA). (Bestas B, et al., 2015)
Fig.1 Proposed treatment options for X-linked agammaglobulinemia (XLA). (Bestas B, et al., 2015)
Recognizing Clinical Suspicion for X-Linked Agammaglobulinemia (XLA)
X-linked agammaglobulinemia (XLA) should be suspected in male infants (6–12 months old) with recurrent or severe bacterial infections, particularly caused by encapsulated organisms, alongside absent tonsils/lymph nodes and failure to thrive. A family history of early male deaths due to infections or immunodeficiency further raises suspicion. These clinical cues, especially poor response to antibiotics or live vaccine complications, should prompt immediate immunological testing to prevent irreversible complications like bronchiectasis. Early recognition is vital, as de novo BTK mutations may occur without familial history.
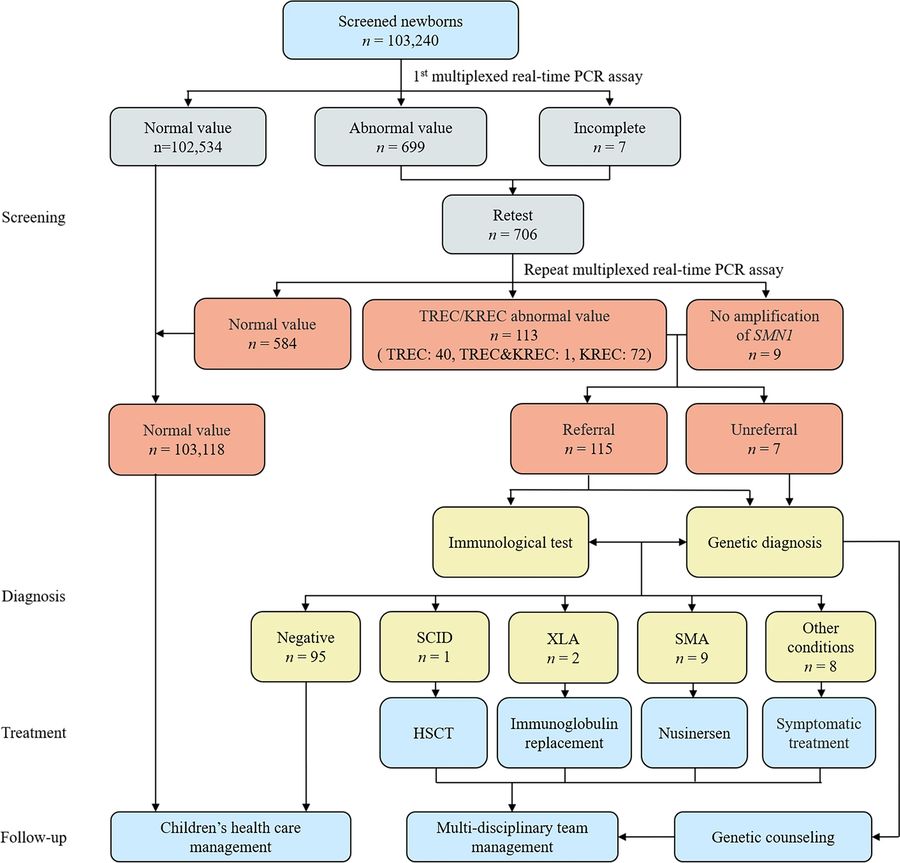 Fig.2 Comprehensive newborn screening for severe combined immunodeficiency, X-linked agammaglobulinemia (XLA), and spinal muscular atrophy (SMA). (Chen C, et al., 2024)
Fig.2 Comprehensive newborn screening for severe combined immunodeficiency, X-linked agammaglobulinemia (XLA), and spinal muscular atrophy (SMA). (Chen C, et al., 2024)
Initial Laboratory Workup for X-Linked Agammaglobulinemia (XLA)
The laboratory diagnosis of X-linked agammaglobulinemia (XLA) aims to confirm hypogammaglobulinemia and B-cell deficiency, which are diagnostic hallmarks of the disease. A stepwise approach includes serum immunoglobulin measurement, B-cell enumeration by flow cytometry, and supportive tests to rule out other immunodeficiencies and assess immune function. Early and accurate testing is crucial to prevent complications from delayed diagnosis.
Serum Immunoglobulins (IgG, IgA, IgM)
XLA patients exhibit severely reduced immunoglobulin levels, with IgG typically < 200 mg/dL and IgA/IgM often near undetectable. Testing must account for age-specific reference ranges, as maternal IgG in infants may temporarily mask deficiency. Repeat testing is recommended if initial results are ambiguous.
B-Cell Enumeration (Flow Cytometry)
Flow cytometry for CD19+ or CD20+ B cells is essential, with XLA patients showing <2% B cells (normal 5–20%). Absent B cells strongly support XLA, but secondary causes (e.g., medication-induced lymphopenia) must be excluded.
Additional Supportive Tests
Supportive tests help strengthen the diagnosis of XLA and rule out mimics. Vaccine antibody titers assess functional antibody production, with XLA patients showing no response despite prior immunization. A complete blood count (CBC) may reveal neutropenia in some cases, while lymphocyte subset analysis confirms the absence of B cells.
Genetic Confirmation of X-Linked Agammaglobulinemia (XLA)
The definitive diagnosis of X-linked agammaglobulinemia (XLA) relies on identification of pathogenic mutations in the BTK (Bruton's tyrosine kinase) gene, located at Xq22.1. Genetic testing not only confirms the diagnosis but also enables family screening, prenatal testing, and differentiation from other forms of agammaglobulinemia.
Targeted BTK Sequencing
The primary method for XLA confirmation, Sanger sequencing efficiently detects small BTK mutations (missense, nonsense, splice-site) in known hotspots (e.g., exons 8, 15). For broader analysis, NGS panels comprehensively scan all BTK exons and flanking regions, identifying ~85% of pathogenic variants.
Deletion/Duplication Analysis
Approximately 10–15% of XLA cases involve large BTK deletions/duplications undetectable by sequencing. MLPA or CNV-seq is essential for these cases, ensuring complete genetic characterization. Always include this step if sequencing is negative but clinical/immunological findings strongly suggest XLA.
The Future of X-Linked Agammaglobulinemia (XLA) Diagnostics
The diagnostic landscape for X-linked agammaglobulinemia (XLA) is evolving with the advent of advanced genetic technologies such as rapid whole genome sequencing (WGS), allowing for faster and more comprehensive BTK mutation analysis. Newborn screening using the TREC assay or BTK protein quantification could aid in early detection before infection develops. AI-driven interpretation tools may improve variant classification, while point-of-care B-cell analysis could streamline initial workup.
Specializing in delivering premium IVD solutions for X-linked agammaglobulinemia (XLA) diagnosis, Alta DiagnoTech offers high-precision immunoassay kits and genetic testing systems to streamline the detection of agammaglobulinemia worldwide. If you have related needs, please feel free to contact us for more information or product support.
References
- Bestas B, Turunen J J, Blomberg K E M, et al. Splice-correction strategies for treatment of X-linked agammaglobulinemia[J]. Current allergy and asthma reports, 2015, 15(3): 4.
- Chen C, Zhang C, Wu D W, et al. Comprehensive newborn screening for severe combined immunodeficiency, X-linked agammaglobulinemia, and spinal muscular atrophy: the Chinese experience[J]. World Journal of Pediatrics, 2024, 20(12): 1270-1282.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Proposed treatment options for X-linked agammaglobulinemia (XLA). (Bestas B, et al., 2015)
Fig.1 Proposed treatment options for X-linked agammaglobulinemia (XLA). (Bestas B, et al., 2015) Fig.2 Comprehensive newborn screening for severe combined immunodeficiency, X-linked agammaglobulinemia (XLA), and spinal muscular atrophy (SMA). (Chen C, et al., 2024)
Fig.2 Comprehensive newborn screening for severe combined immunodeficiency, X-linked agammaglobulinemia (XLA), and spinal muscular atrophy (SMA). (Chen C, et al., 2024)