- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Whooping Cough Diagnostic Methods Explained: PCR, Serology, and Key Biomarkers
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Accurate diagnosis of whooping cough remains challenging due to evolving strains, variable symptom presentation, and limitations of individual testing methods. This resource provides healthcare professionals and laboratory specialists with a detailed examination of current and emerging diagnostic approaches, including molecular (PCR), serological (anti-PT IgG), and culture-based methods, analyzing their performance characteristics and practical limitations across different disease stages.
Whooping cough, caused by the bacterium Bordetella pertussis, is a highly contagious respiratory infection characterized by severe coughing fits followed by a distinctive "whooping" sound during inhalation. Primarily affecting infants and young children, it remains a significant global health concern with an estimated 24 million cases annually, despite widespread vaccination. The disease progresses through three stages: catarrhal (cold-like symptoms), paroxysmal (intense coughing), and convalescent (gradual recovery).
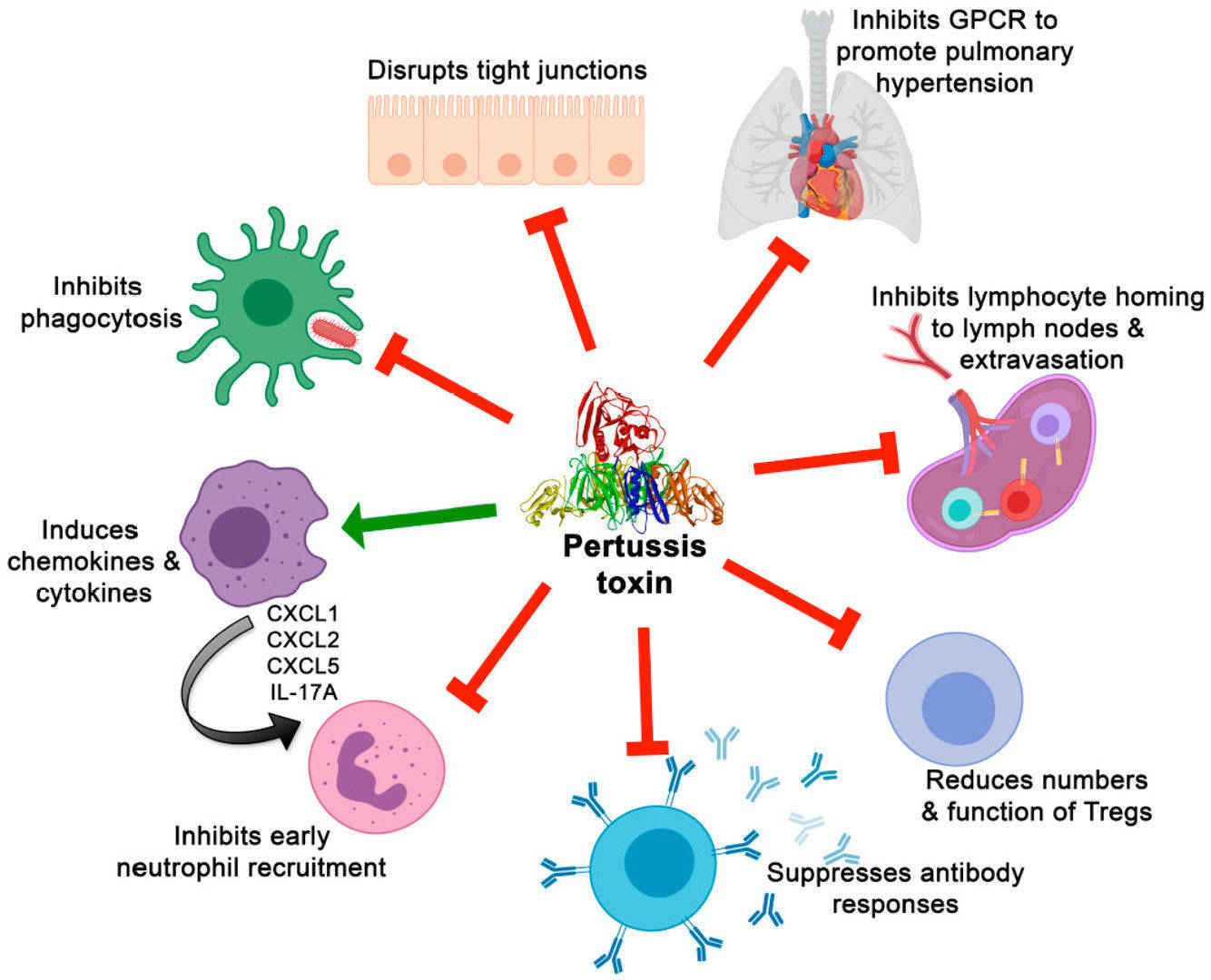 Fig.1 Effects of pertussis toxin on immune cells and responses and on other aspects of pertussis pathogenesis. (Scanlon K, et al., 2019)
Fig.1 Effects of pertussis toxin on immune cells and responses and on other aspects of pertussis pathogenesis. (Scanlon K, et al., 2019)
Given that whooping cough is extremely contagious, accurate and timely diagnosis of pertussis is critical to preventing severe outcomes in vulnerable populations, especially unvaccinated infants, and controlling outbreaks. Diagnosis relies on PCR testing (early), serological testing (late), and Bordetella pertussis culture, and vaccination status can significantly affect the interpretation of test results.
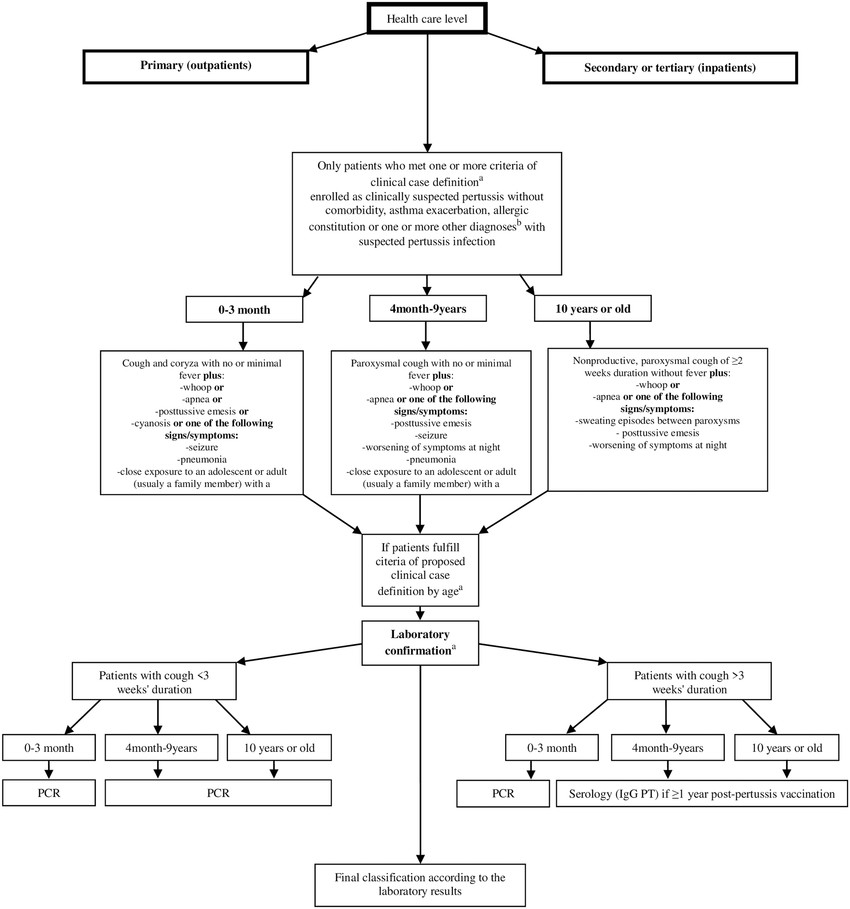 Fig.2 Algorithm for the surveillance and laboratory diagnosis of pertussis. (Ristić M, et al., 2018)
Fig.2 Algorithm for the surveillance and laboratory diagnosis of pertussis. (Ristić M, et al., 2018)
Three primary diagnostic approaches, PCR, serology, and bacterial culture, each play distinct roles in whooping cough detection, with selection dependent on symptom timing, clinical context, and laboratory capabilities.

PCR is the gold standard for early detection (first 2–3 weeks of illness), targeting B. pertussis-specific DNA sequences (e.g., IS481 and PT toxin genes). It offers high sensitivity (90–95%) and rapid turnaround (hours), making it critical for outbreak investigations and infant cases. However, PCR cannot distinguish between active infection and residual DNA post-vaccination or past infection.

Serology detects anti-pertussis toxin (PT) IgG antibodies and is most useful after the third week of illness, when PCR sensitivity declines. It helps confirm late-stage or missed diagnoses, particularly in adolescents and adults. However, interpretation is complicated by prior vaccination (DTaP/Tdap), which can cause false positives.

Though highly specific (100%), culture has low sensitivity (30–50%) and requires specialized media and prolonged incubation (7–10 days). It remains valuable for antibiotic susceptibility testing and strain surveillance but is impractical for routine diagnosis. Culture is most effective when performed early (catarrhal phase) and with proper nasopharyngeal aspirate/swab collection.
Accurate diagnosis of whooping cough (pertussis) relies on detecting specific biomarkers produced by Bordetella pertussis, the causative bacterium. These biomarkers help differentiate active infection from vaccination effects or other respiratory illnesses. Below, we discuss the key molecular and immunological markers used in laboratory testing.
Genomic Biomarkers
Genomic biomarkers, such as IS481 and PT toxin genes, are ideal for early diagnosis (1-2 weeks). Although IS481 has broad sensitivity, it lacks specificity due to cross-reactivity with B. holmesii. In contrast, PT toxin genes are species-specific but require optimized assays due to low abundance.
Protein Biomarkers
Protein biomarkers, particularly pertussis toxin (PT), can be used serologically to confirm late infection (3 weeks or more after infection). Anti-PT IgG is the most specific serological marker, but its utility is limited in vaccinated individuals due to vaccine-induced antibodies.
Current diagnostic methods for whooping cough face significant limitations, including the narrow optimal testing window for PCR (first 2-3 weeks), serological interference from vaccination, and poor sensitivity of bacterial culture. Emerging solutions focus on developing rapid point-of-care molecular tests, multiplex assays to differentiate pertussis from similar infections, and novel biomarkers to distinguish natural infection from vaccine response.
Alta DiagnoTech offers approved in vitro diagnostic (IVD) products for whooping cough, such as PCR kits and anti-pertussis toxin (PT) antibody test kits, ensuring accurate and compliant testing across healthcare settings. If you have related needs, please feel free to contact us for more information or product support.
References
| Cat.No | Product Name | Price |
|---|---|---|
| EK-YJL-3915 | Human Bordetella Pertussis Antibody (IgM) ELISA Kit | Add To Cart |
| NATR-HMM-0039 | Bordetella pertussis Nucleic Acid Detection Reagent (TaqMan qPCR) | Add To Cart |
| BI-QCY-0002 | Bordetella Infection PCR Test Kit | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |