- Home
- Resource
- Disease Diagnosis
- Genetic Diseases
- Decoding Hemophilia: Modern Diagnostic Approaches & Key Biomarkers
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Hemophilia, a complex inherited bleeding disorder, demands precise diagnosis to guide effective treatment and prevent complications. This resource explores the essential diagnostic workflow, from initial clinical suspicion to advanced biomarker analysis, while highlighting cutting-edge technologies reshaping detection and monitoring.
Hemophilia is a rare, inherited bleeding disorder caused by deficiencies in clotting factor VIII (Hemophilia A) or factor IX (Hemophilia B), leading to impaired blood coagulation. Primarily affecting males due to its X-linked recessive inheritance, hemophilia is classified by severity, which varies from mild to severe, based on residual factor activity levels. Common symptoms include prolonged bleeding after injuries, spontaneous joint bleeds, and excessive post-surgical hemorrhage.
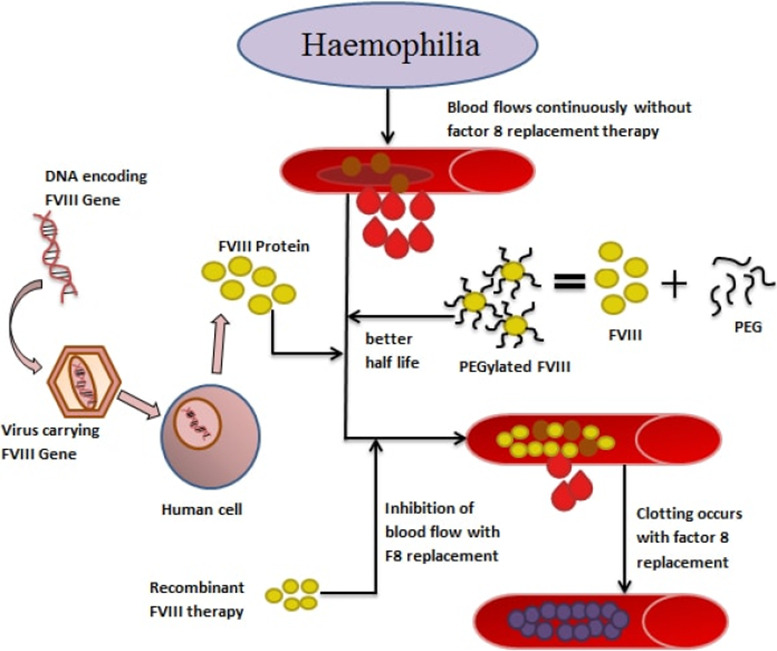 Fig.1 Advancement in the treatment of hemophilia. (Bhardwaj R, et al., 2018)
Fig.1 Advancement in the treatment of hemophilia. (Bhardwaj R, et al., 2018)
The accurate diagnosis of hemophilia is critical for preventing life-threatening bleeding complications, minimizing long-term joint damage, and guiding personalized treatment strategies such as factor replacement therapy or gene therapy. Early diagnosis, particularly in infants with a family history, helps initiate prophylactic care, improving quality of life and reducing disability. Testing should be pursued in cases of unexplained prolonged bleeding, spontaneous joint/muscle hemorrhages, abnormal coagulation screening (e.g., isolated prolonged aPTT), or a family history of hemophilia. Timely diagnosis also enables genetic counseling for carriers and informs surgical or obstetric planning to avoid bleeding risks.
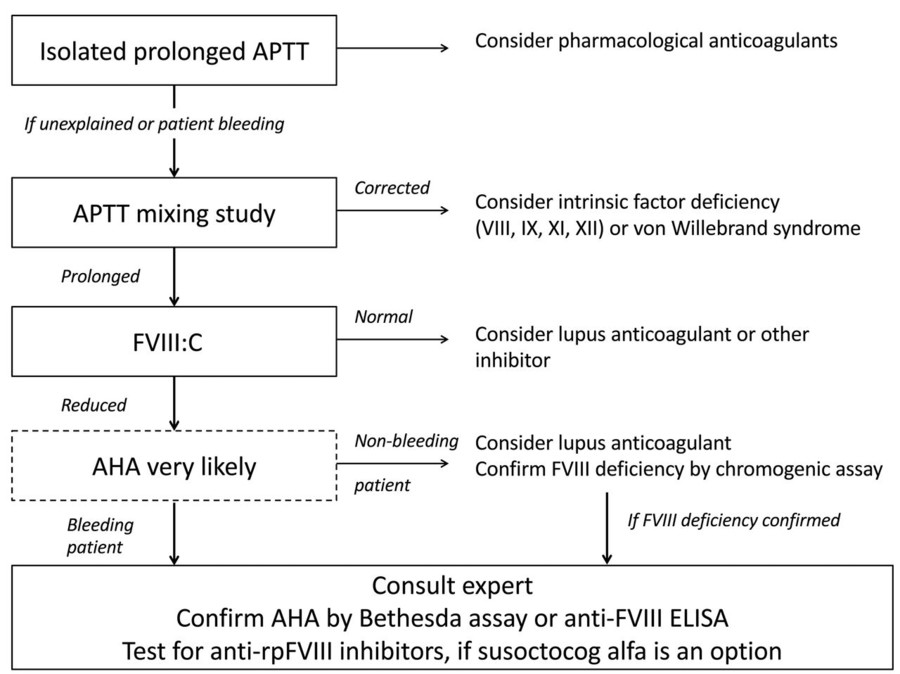 Fig.2 Diagnostic pathway for acquired hemophilia A (AHA). (Tiede A, et al., 2020)
Fig.2 Diagnostic pathway for acquired hemophilia A (AHA). (Tiede A, et al., 2020)
The diagnosis of hemophilia follows a stepwise laboratory approach to confirm clotting factor deficiencies and rule out other bleeding disorders. Here's a structured workflow:

Initial Clinical Suspicion
The diagnostic journey begins when patients present with unexplained bleeding symptoms or have a family history of hemophilia. Neonates with abnormal umbilical bleeding or post-circumcision hemorrhage should raise immediate suspicion, as should isolated prolonged aPTT found incidentally on coagulation screens.
First-Line Screening Tests
Initial lab workup focuses on aPTT (typically prolonged except in mild hemophilia), PT (normal, ruling out other coagulopathies), and fibrinogen testing. An isolated prolonged aPTT with normal PT/INR strongly suggests factor VIII/IX deficiency and warrants confirmatory testing.
Confirmatory Factor Assays
Definitive diagnosis requires quantitative factor activity measurement (chromogenic or one-stage assays) to distinguish hemophilia A (FVIII deficiency) from B (FIX deficiency) and classify severity. Concurrent von Willebrand factor testing helps exclude type 2N VWD mimicking hemophilia A.
Additional Testing
Specialized tests include mixing studies (to identify inhibitors), Bethesda assays (quantify inhibitor titers), and genetic testing (F8/F9 gene analysis for carriers/prenatal diagnosis). Global assays like thrombin generation may provide supplementary data.

Differential Diagnosis
Key conditions to rule out include von Willebrand disease (normal factor VIII with abnormal VWF), platelet disorders (normal coagulation tests), acquired hemophilia (sudden FVIII deficiency with inhibitors), and rare factor deficiencies (e.g., XI, XIII). Clinical context guides this exclusion process.
Hemophilia diagnosis and management rely on a combination of classic coagulation biomarkers and emerging indicators that provide insights into disease severity, treatment response, and complications.
Conventional Biomarkers
Conventional biomarkers remain the cornerstone of hemophilia diagnosis and monitoring, including factor VIII/IX activity assays (gold standard for classification and severity grading), aPTT (screening for coagulation defects), and inhibitor detection (Bethesda assay for treatment guidance). These established tools are complemented by von Willebrand factor (VWF) testing to exclude type 2N VWD, which mimics hemophilia A.
Emerging Biomarkers
Innovative biomarkers are transforming hemophilia management, with thrombin generation assays (TGA) providing a global assessment of clotting potential beyond isolated factor levels. Research explores microRNAs for predicting inhibitor development, inflammatory markers (IL-6, TNF-α) linked to arthropathy progression, and genetic profiling (F8/F9 mutations) to personalize therapies like gene editing.
Despite advances, hemophilia diagnosis faces hurdles like false-negative screenings in mild cases, limited access to specialized factor assays in resource-limited settings, and inhibitor interference complicating treatment monitoring. Emerging solutions include point-of-care genetic testing, AI-powered coagulation analysis, and CRISPR-based diagnostics for rapid mutation detection. The future lies in non-invasive biomarkers (e.g., microRNAs) and personalized algorithms integrating multi-omics data to predict bleeding risks and optimize therapy, ultimately enabling earlier, more precise global diagnosis.
Alta DiagnoTech offers a complete portfolio of hemophilia diagnostic products, from rapid screening kits to confirmatory factor assays, all developed using cutting-edge technology to ensure optimal accuracy and reliability for each test. If you have related needs, please feel free to contact us for more information or product support.
References
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |