- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Chlamydia Diagnostics: The 2025 European Guideline for Accurate & Actionable Testing
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Accurate and timely diagnosis of Chlamydia, the most prevalent bacterial STI, is critical to preventing complications like infertility and curbing silent transmission. This resource synthesizes the 2025 European Guideline into actionable strategies for laboratories and clinicians to enhance detection accuracy, streamline workflows, and combat asymptomatic transmission in line with current standards.
Chlamydia is the most prevalent bacterial sexually transmitted infection (STI) globally, with a significant public health burden due to its frequently asymptomatic nature. Up to 70% of cases are asymptomatic, leading to hidden transmission and complications such as pelvic inflammatory disease (PID), infertility, and ectopic pregnancy. The 2025 European Guideline underscores the critical role of accurate, NAAT-based diagnostics in early detection and management, particularly for high-risk groups (e.g., women under 25, MSM). Standardized testing pathways are essential to curb transmission and align with evolving antimicrobial resistance concerns.
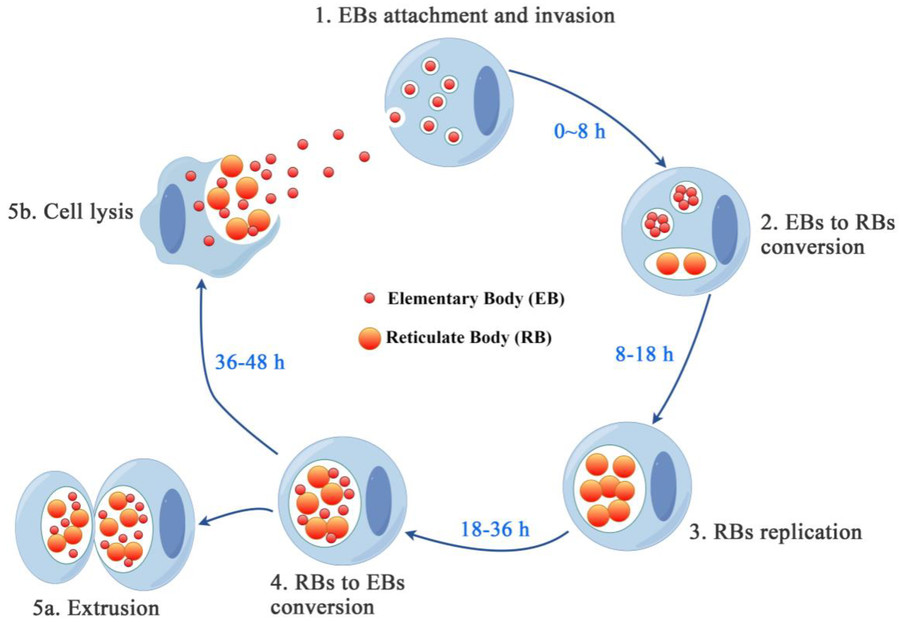 Fig.1 Chlamydia developmental cycle. (Yang S, et al., 2024)
Fig.1 Chlamydia developmental cycle. (Yang S, et al., 2024)
✔ Superior Accuracy: Detects even low bacterial loads in asymptomatic cases.
✔ Broad Sample Compatibility: Validated for first-void urine (FVU), vaginal/cervical swabs (self- or clinician-collected), and extragenital samples (rectal/pharyngeal).
✔ Automation & Scalability: High-throughput platforms enable efficient large-scale screening.
The gold standard for diagnosing Chlamydia trachomatis infections, as established by the 2025 European Guideline, is Nucleic Acid Amplification Testing (NAAT). NAATs are the most sensitive (>99%) and specific (>98%) method for detecting C. trachomatis, outperforming traditional techniques like culture, enzyme immunoassays (EIAs), or rapid antigen tests.
Accurate diagnosis of Chlamydia trachomatis infections requires a systematic approach aligned with the 2025 European Guideline. Below is a clear, actionable testing algorithm for clinicians and laboratories:
Screen asymptomatic high-risk groups (women <25, MSM, pregnant women) and test symptomatic patients (dysuria, discharge, PID). Annual screening for at-risk populations is critical due to frequent asymptomatic infections (70% cases).
| Patient Population | Recommended Sample | Notes |
| Women | Vaginal/cervical swab (self- or clinician-collected) | Self-swabs show equal sensitivity to clinician-collected |
| Men | First-void urine (FVU) | ≥20 mL, initial stream |
| MSM/Extragenital Exposure | Rectal/pharyngeal swab | Must use NAATs validated for extragenital sites |
| Pregnant Women | Vaginal swab or FVU | Avoid cervical swabs if cervical incompetence risk |
The 2025 European Guideline mandates NAATs (PCR, TMA, or SDA) as the exclusive testing method due to their superior sensitivity (>99%) and specificity (>98%) compared to outdated EIA/rapid antigen tests. Laboratories must:
(1) Use CE-IVD marked NAATs validated for all sample types (genital/extra-genital);
(2) Prioritize automated high-throughput platforms for efficient screening;
(3) Implement strict pre-analytical controls (e.g., urine volume ≥20mL, swab transport within 72h at 2-8℃);
(4) Consider multiplex NAATs for simultaneous N. gonorrhoeae detection in symptomatic cases.
Crucially, extragenital samples (rectal/pharyngeal) require separate validation per EU regulations, as 15-25% of MSM infections are missed by urogenital testing alone. Internal QC should monitor for PCR inhibitors in urine samples, while avoiding cross-contamination in batch processing.
A positive NAAT result requires immediate treatment (e.g., doxycycline or azithromycin) and partner notification/testing within a 60-day window. Test-of-cure (TOC) is only recommended for pregnant women or suspected treatment failure, performed ≥3 weeks post-treatment to avoid false positives from residual DNA. Negative results in high-risk individuals should trigger retesting in 3–6 months due to potential exposure. Asymptomatic cases must still be treated to prevent complications and transmission. Follow-up emphasizes partner management and risk-reduction counseling, as reinfection rates exceed 20% within a year.
The field of Chlamydia trachomatis diagnostics is undergoing rapid transformation, driven by advances in molecular technology and shifting healthcare demands. Three key innovations are reshaping testing paradigms:

Portable molecular devices now provide NAAT-level accuracy (95–99% sensitivity) in <60 minutes, eliminating the need for centralized labs. Some point-of-care testing platforms can be used in STI clinics, emergency rooms, and pharmacies, supporting same-day testing and treatment. The 2025 EU guideline highlights their potential for high-risk populations where delayed results often lead to loss-to-follow-up.

Self-collection kits paired with telehealth platforms have demonstrated 95% concordance with clinic-based NAATs. Dried sample technologies (e.g., urine filter paper) facilitate stable mail-in testing, particularly valuable for rural/young populations. The EU recommends these solutions to address screening barriers but emphasizes provider-mediated interpretation of results to ensure appropriate treatment.
Next-gen NAATs simultaneously detect C. trachomatis, N. gonorrhoeae, M. genitalium, and T. vaginalis from a single swab, streamlining syndromic management. Some assays integrate antibiotic resistance markers (e.g., macrolide resistance in M. genitalium), aligning with WHO AMR surveillance goals. The 2025 guideline cautions against overuse in low-prevalence settings but endorses them for symptomatic patients and MSM.
Alta DiagnoTech delivers high-accuracy, guideline-compliant IVD solutions for Chlamydia, combining NAAT-powered precision with user-friendly workflows. If you have related needs, please feel free to contact us for more information or product support.
References
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |