- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Breaking Down Ebola Virus Disease (EVD) Diagnostics: Methods, Biomarkers & Future Frontiers
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Ebola virus disease (EVD) is a severe, often fatal hemorrhagic fever caused by the Ebola virus. Accurate and timely diagnosis of EVD is essential for effective patient management and outbreak containment. This resource provides a comprehensive overview of current diagnostic methods, from gold-standard molecular tests to rapid point-of-care solutions, along with key biomarkers and technical challenges.
Ebola virus disease (EVD) is a severe, often fatal illness caused by the Ebola virus, with mortality rates reaching up to 90% in some outbreaks. Primarily transmitted through direct contact with bodily fluids of infected individuals or animals, EVD is characterized by sudden onset of fever, fatigue, and hemorrhagic symptoms. Its high contagion risk and potential for rapid spread during outbreaks underscore the critical need for early and accurate diagnostics. Timely detection not only improves patient outcomes but also enables effective containment, making diagnostic tools a cornerstone of global health preparedness against this deadly pathogen.
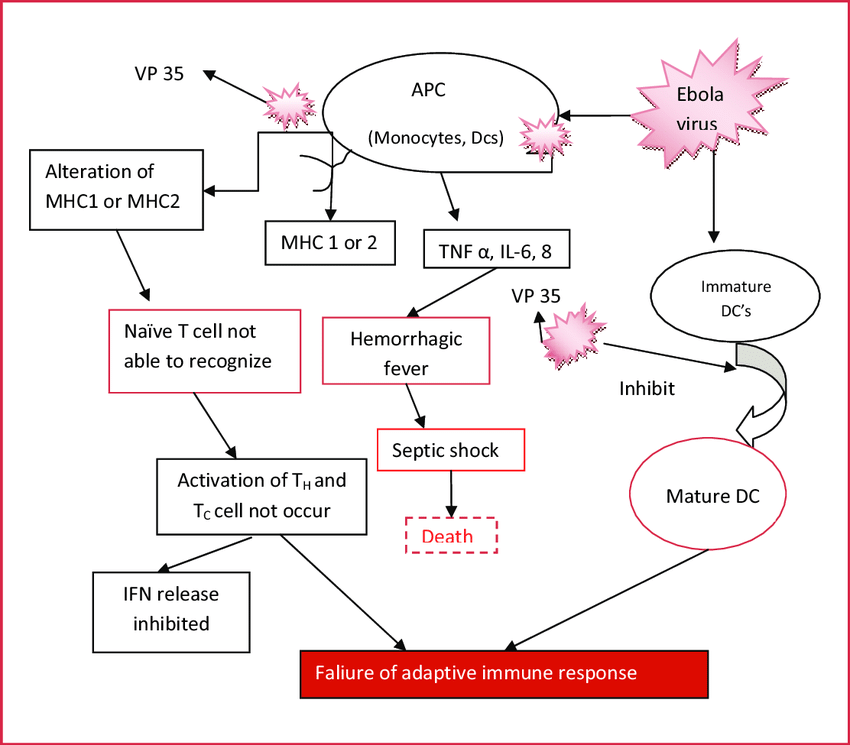 Fig.1 Pathogenesis of Ebola virus disease (EVD). (Singh Jadav S, et al., 2015)
Fig.1 Pathogenesis of Ebola virus disease (EVD). (Singh Jadav S, et al., 2015)
Accurate and timely diagnosis of Ebola virus disease (EVD) is critical for outbreak control and patient management. The primary diagnostic approaches include molecular tests, antigen-based rapid tests, and serological tests.

Molecular tests, particularly reverse transcription polymerase chain reaction (RT-PCR), are the most reliable for detecting Ebola virus RNA during acute infection. These tests target viral genes (e.g., NP, GP) with high sensitivity (>95%) as early as 3 days post-symptom onset. While highly accurate, they require BSL-4 lab facilities and specialized equipment, limiting use in resource-poor settings.

Lateral flow assays (LFAs) detect Ebola viral proteins (e.g., VP40) and provide results in 15–30 minutes, making them ideal for field triage and outbreak settings. While less sensitive (~70–90%) than PCR, they are cost-effective, require minimal training, and do not need cold storage. Rapid tests are most effective 3+ days post-symptom onset and should be confirmed by PCR when possible.
These tests detect IgM and IgG antibodies, which appear 7–10 days after symptom onset, making them useful for surveillance, recovery monitoring, and epidemiological studies. ELISA is the most common format, but cross-reactivity with other filoviruses can be a limitation. Serology is not suitable for acute diagnosis but helps identify past infections and assess immune responses in survivors.
Effective diagnosis of Ebola virus disease (EVD) relies on detecting specific viral and host biomarkers that indicate infection, disease progression, and immune response. These biomarkers help differentiate EVD from other febrile illnesses, assess disease severity, and guide clinical management.

Viral Biomarkers
Viral biomarkers directly detect Ebola virus components, confirming active infection. Key targets include viral RNA (NP, GP, and VP40 genes) for RT-PCR assays and VP40 protein for rapid antigen tests. These biomarkers offer high specificity and are most reliable from Day 3 post-symptom onset, making them critical for early diagnosis and outbreak containment.
Host Biomarkers
Host biomarkers reflect the body's response to infection, aiding in severity assessment and prognosis. These include elevated cytokines (IL-6, IFN-γ), thrombocytopenia, and liver enzyme abnormalities (AST/ALT). Antibodies (IgM/IgG) appear later (Day 7+) and help confirm past infections or immune responses, though they lack utility for acute diagnosis. Host biomarkers complement viral testing by guiding clinical management and predicting outcomes.
Ebola virus disease (EVD) diagnostics face significant hurdles, including limited early detection sensitivity (low viral loads pre-Day 3), biosafety requirements (BSL-4 labs for live virus handling), and infrastructure gaps in resource-limited outbreak settings. Additional challenges include cross-reactivity in serological tests, cold-chain dependence for molecular assays, and the need for rapid, deployable tools to curb outbreaks effectively. Overcoming these barriers is critical to improving diagnostic accuracy, accessibility, and timely response.
To overcome the diagnostic challenges of Ebola virus disease (EVD), Alta DiagnoTech provides cutting-edge IVD products to facilitate effective disease management. Our extensive product line includes reagents, kits, devices and consumables. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| VT-QCY-0013 | Tai Forest Ebola Virus RT-PCR Kit | Add To Cart |
| VT-QCY-0011 | Zaire Ebola Virus PCR Kit | Add To Cart |
| PK-QCY-0006 | Rapid Plant Genotyping Assay Kit | Add To Cart |
| PK-QCY-0007 | Multiplex PCR Kit | Add To Cart |
| PK-QCY-0009 | E.coli DNA Residue Probe qPCR Kit | Add To Cart |
| PK-QCY-0003 | Animal Tissue Direct PCR Kit | Add To Cart |
| PK-QCY-0002 | E.coli Colony Direct PCR Kit | Add To Cart |
| PK-QCY-0004 | Mouse Tail Quick Genotyping Assay Kit | Add To Cart |
| PK-QCY-0001 | Adenovirus-type PCR Test Kit | Add To Cart |
| PK-QCY-0011 | Human Telomere Length SYBR Green qPCR Kit | Add To Cart |
| PK-QCY-0010 | Novel Coronavirus Dual Probes qRT-PCR Kit | Add To Cart |
| PK-QCY-0008 | Random Mutagenesis PCR Kit | Add To Cart |
| PK-QCY-0005 | Rodents One-step Genotyping Assay Kit | Add To Cart |
| P-001 | qPCR Mix kit | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |