- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- The Evolution of Chickenpox Diagnostics: Integrating PCR, Serology & Point-of-Care Innovations
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Chickenpox, caused by the varicella-zoster virus (VZV), remains a global health concern despite vaccine availability. This comprehensive resource describes traditional laboratory testing methods and modern diagnostic strategies for varicella. It is designed to provide healthcare professionals with essential knowledge about PCR testing, serological assays, and point-of-care diagnostic solutions to improve diagnostic accuracy in various clinical scenarios.
Chickenpox, caused by the varicella-zoster virus (VZV), is a highly contagious disease characterized by itchy vesicular rashes, fever, and malaise. While typically self-limiting in healthy children, it can cause severe complications in high-risk groups (immunocompromised individuals, pregnant women, and neonates). Diagnosis traditionally relies on clinical presentation, but atypical cases (e.g., breakthrough varicella in vaccinated patients) and differential diagnoses (e.g., HSV, enterovirus) often require laboratory confirmation.
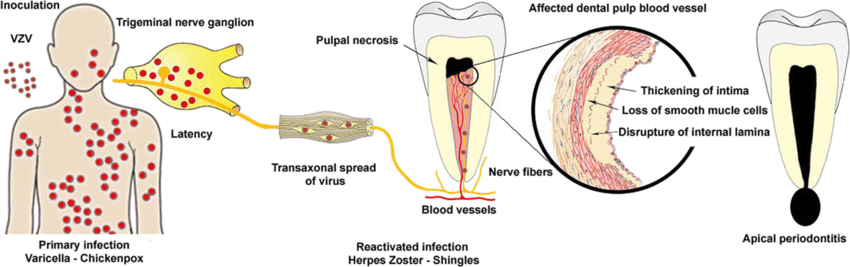 Fig.1 Mechanism of varicella zoster virus (VZV)-induced vasculitis of dental pulp blood vessels. (Jakovljevic, Aleksandar, et al., 2018)
Fig.1 Mechanism of varicella zoster virus (VZV)-induced vasculitis of dental pulp blood vessels. (Jakovljevic, Aleksandar, et al., 2018)
Chickenpox (varicella) has historically been diagnosed through a combination of clinical evaluation and laboratory techniques, each with distinct strengths and limitations. While newer molecular methods now dominate, traditional approaches remain relevant in resource-limited settings or for retrospective analysis.

Chickenpox is typically diagnosed clinically by its characteristic rash (pruritic vesicles in crops) and systemic symptoms (fever, malaise). While reliable for classic cases, it struggles with atypical presentations (breakthrough varicella, immunocompromised patients) and mimics (HSV, enterovirus), necessitating lab confirmation in high-risk scenarios.

DFA detects VZV antigens in vesicle scrapings via fluorescent antibodies, offering rapid results (1–2 hours). Though faster than culture, its moderate sensitivity (~70–80%) and declining use make it a backup option where PCR is unavailable.

Once the gold standard, culture isolates live virus from lesions/cerebrospinal fluid (CSF) but suffers from low sensitivity (<60%) and slow turnaround (3–7 days). Now largely obsolete clinically, it persists only for research or antiviral resistance testing.
While traditional methods like clinical assessment and DFA remain useful, modern laboratory techniques have revolutionized varicella-zoster virus (VZV) diagnostics, offering superior accuracy, speed, and clinical utility. These advanced methods are particularly critical for atypical cases, high-risk patients, and immunity status determination.
PCR (Gold Standard)
PCR is the most accurate method for active VZV infection, detecting viral DNA in vesicle swabs, blood, or CSF with >98% sensitivity within hours. It is critical for diagnosing atypical cases (e.g., breakthrough varicella, CNS infections) and differentiating VZV from HSV or enteroviruses via multiplex assays.
Serology (IgM/IgG)
Serology identifies VZV-specific antibodies, with IgM indicating recent infection and IgG confirming immunity (e.g., pre-vaccination or prenatal screening). While useful for immunity assessment, IgM may lag behind symptoms, and IgG cannot distinguish vaccine from wild-type strains.
Point-of-care (POC) technologies are transforming varicella-zoster virus (VZV) diagnostics by enabling rapid, on-site testing without specialized lab infrastructure. These innovations address critical gaps in timely diagnosis, particularly for emergency departments, pediatric clinics, and resource-limited settings. Below are key advancements in POC solutions for chickenpox:
Portable PCR platforms deliver lab-quality VZV detection in <1 hour through automated, cartridge-based workflows, enabling same-visit diagnosis in ERs or clinics without specialized lab infrastructure.
These disposable strips detect VZV antigens from vesicle swabs in 15–30 minutes, offering ultra-fast triage for outbreaks or resource-limited settings, though with lower sensitivity (~75%) than PCR.
The future of varicella-zoster virus (VZV) diagnostics lies in ultra-rapid, accessible, and intelligent technologies, including home tests for equipment-free detection, AI-integrated smartphone apps for rash analysis with lab-grade accuracy, and microfluidic "lab-on-a-chip" devices combining multiplex PCR with resistance profiling. All aimed at enabling real-time decision-making, personalized treatment, and global surveillance of varicella outbreaks.
To support efficient chickenpox management, Alta DiagnoTech offers a comprehensive range of precise IVD solutions, including rapid PCR test kits, multiplex assay kits, and point-of-care diagnostic tools for accurate VZV detection and immunity assessment. If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |