- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- The Complete Guide to Tuberculosis (TB) Diagnostic Pathways: Techniques & Key Biomarkers
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Tuberculosis (TB) remains one of the world's deadliest infectious diseases, with millions of new cases reported annually. Accurate and timely diagnosis is critical to controlling transmission, initiating proper treatment, and combating drug-resistant strains. This resource provides a comprehensive overview of modern TB diagnostic approaches, from conventional methods to advanced molecular techniques and biomarker-based strategies.
Tuberculosis (TB), caused by Mycobacterium tuberculosis, remains a leading global infectious killer, with an estimated 10 million cases and 1.5 million deaths annually. Primarily affecting the lungs (pulmonary TB), it can also invade other organs (extrapulmonary TB), posing diagnostic challenges. TB spreads through airborne droplets, disproportionately impacting immunocompromised individuals and regions with limited healthcare access.
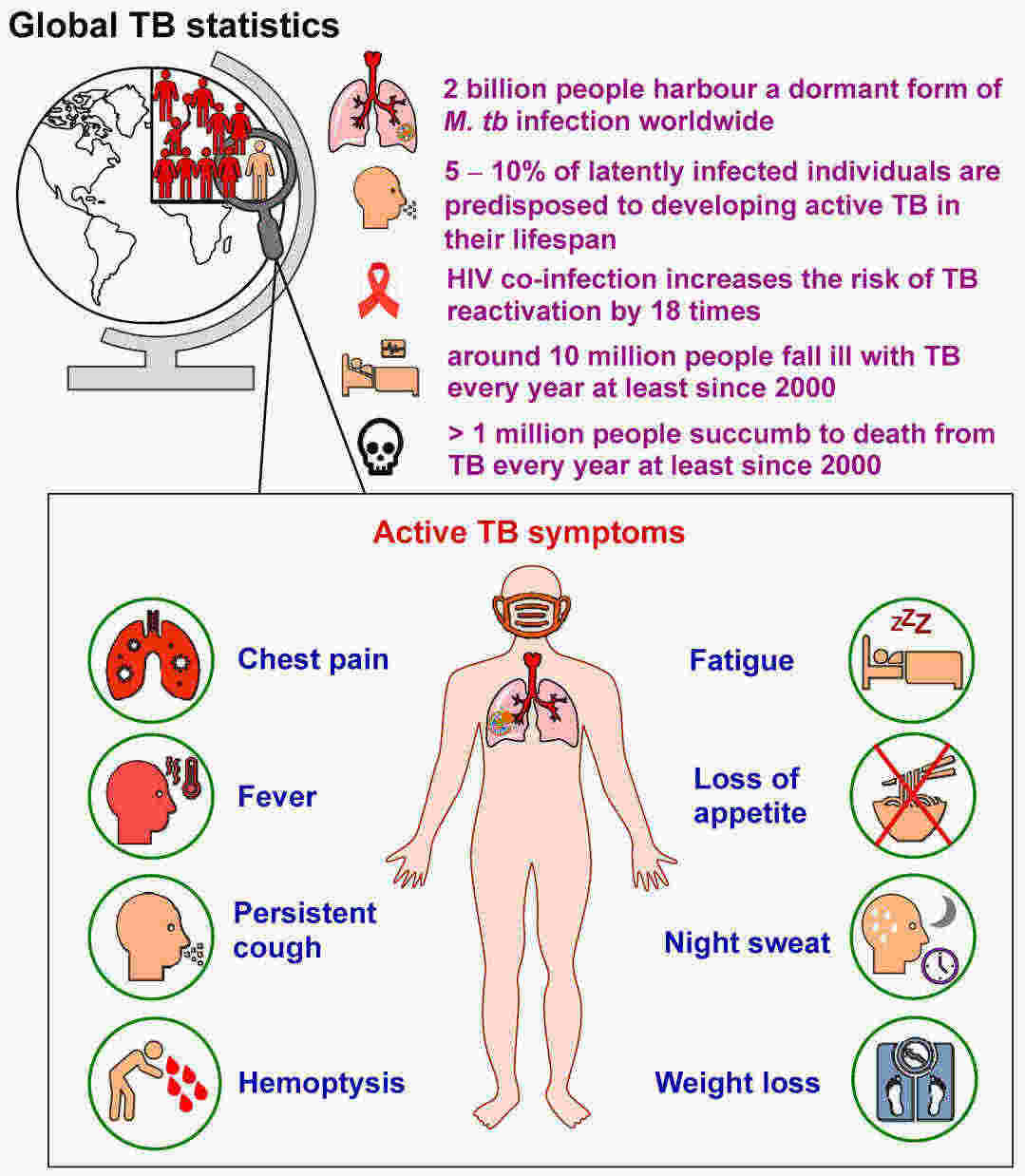 Fig.1 General tuberculosis (TB) statistics and main symptoms of pulmonary TB. (Alsayed, Shahinda SR, and Hendra Gunosewoyo., 2023)
Fig.1 General tuberculosis (TB) statistics and main symptoms of pulmonary TB. (Alsayed, Shahinda SR, and Hendra Gunosewoyo., 2023)
Accurate and timely diagnosis plays a pivotal role in preventing the transmission of tuberculosis (TB), lowering mortality rates, and combating drug resistance. Despite the challenges posed by nonspecific symptoms, traditional testing limitations, and the intricacies of high-risk populations, establishing a structured diagnostic workflow is key. Such a framework not only facilitates efficient testing but also minimizes delays and optimizes resource allocation.
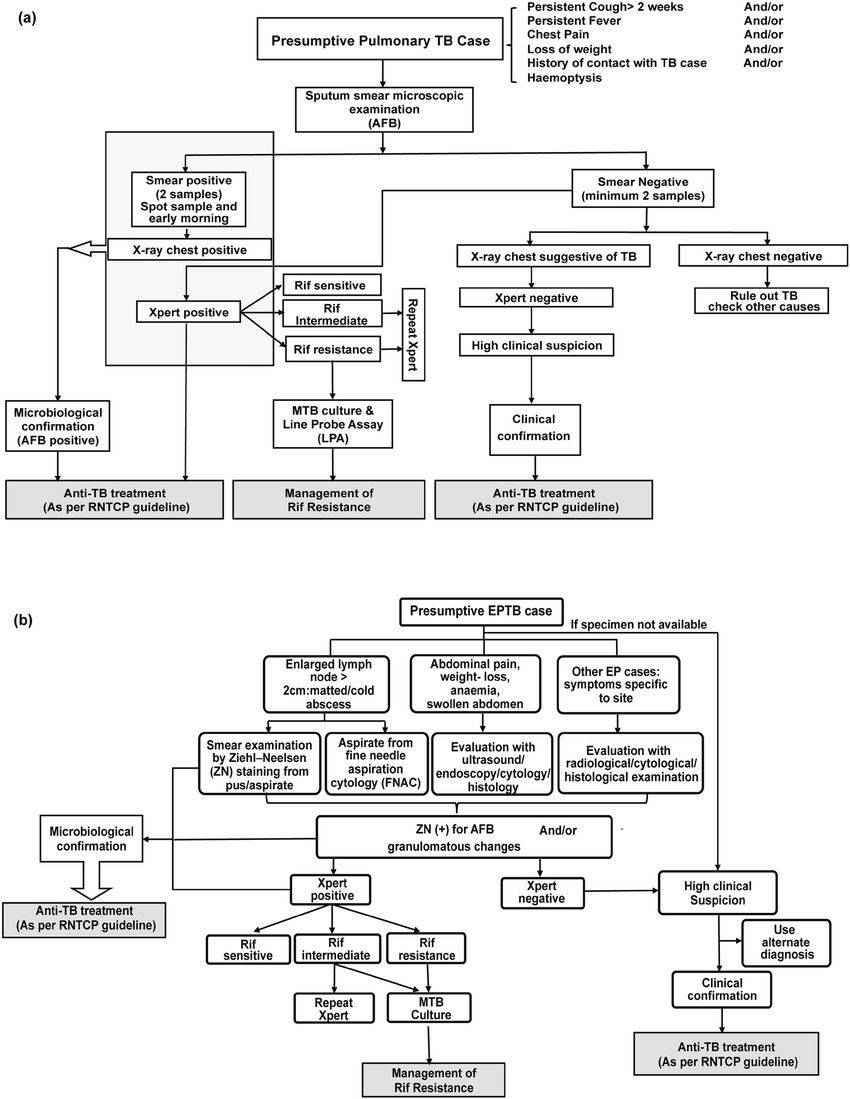 Fig.2 The tuberculosis (TB) diagnostic algorithm proposed by the Revised National TB Control Program (RNTCP) guidelines. (Gaur, Mohita, et al., 2020)
Fig.2 The tuberculosis (TB) diagnostic algorithm proposed by the Revised National TB Control Program (RNTCP) guidelines. (Gaur, Mohita, et al., 2020)
Tuberculosis (TB) diagnosis requires high sensitivity and specificity to address its global health burden, drug resistance, and diverse clinical presentations. Diagnostic methods range from traditional techniques (e.g., smear microscopy) to advanced molecular assays, each with distinct advantages and limitations.
Sputum Smear Microscopy
A rapid, low-cost method that visually detects acid-fast bacilli in sputum samples but has limited sensitivity (~50%) and cannot distinguish M. tuberculosis from other mycobacteria.
Tuberculin Skin Test (TST)
An intradermal test measuring delayed hypersensitivity to tuberculin (PPD), widely used for latent TB screening but prone to false positives due to BCG vaccination or non-TB mycobacterial exposure.
Solid & Liquid Culture
The gold standard for TB diagnosis, offering high sensitivity and enabling drug susceptibility testing, but requiring 2–6 weeks for results and specialized lab infrastructure.

POC tests enable rapid TB diagnosis at the patient's side, particularly crucial in resource-limited settings. These portable, easy-to-use devices (e.g., lateral flow urine LAM tests and handheld molecular systems) provide results within hours, bypassing lab dependency. While offering advantages in accessibility and speed, most POC tests currently trade off some sensitivity compared to centralized lab methods, especially in smear-negative or HIV-coinfected cases. Recent advances aim to improve accuracy while maintaining simplicity.
Tuberculosis (TB) biomarkers are critical for improving diagnostic accuracy, especially in challenging cases such as paucibacillary, pediatric, and extrapulmonary TB. These biomarkers include pathogen-derived components and host immune response signatures. Their development aims to overcome limitations of traditional methods by enabling faster, non-sputum-based, and more precise TB detection.

These biomarkers directly detect components of Mycobacterium tuberculosis, offering high specificity for TB diagnosis. Key examples include lipoarabinomannan (LAM) antigen in urine, bacterial DNA targets like IS6110 for PCR tests, and secreted proteins such as ESAT-6/CFP-10 used in IGRA assays. These markers are particularly valuable for active TB detection and drug resistance profiling.

These biomarkers measure the human immune system's reaction to TB infection, including cytokine patterns (IFN-γ, IP-10), antibody responses, and gene expression signatures. They help differentiate active TB from latent infection or other diseases, and show promise for non-sputum based diagnosis. While less specific than pathogen markers, they can reflect disease progression and treatment response.

Innovative approaches include breath-based volatile organic compounds (VOCs), metabolic profiles, and multi-analyte signatures combining pathogen and host markers. These next-generation biomarkers aim to overcome current limitations by enabling non-invasive sampling (breath, urine), improving detection of paucibacillary cases, and facilitating point-of-care testing.
Despite advancements, tuberculosis (TB) diagnosis still faces significant hurdles, including the low sensitivity of conventional tests, lengthy turnaround times for culture, and difficulties in detecting paucibacillary, pediatric, and extrapulmonary TB. Additionally, the rise of drug-resistant strains (MDR/XDR-TB) demands more sophisticated diagnostics. Future solutions focus on non-sputum-based biomarkers, point-of-care molecular tests, and artificial intelligence-assisted tools to improve speed, accuracy, and accessibility—particularly in high-burden, resource-limited settings. Innovations in multi-omics approaches and portable sequencing technologies also hold promise for personalized TB management and outbreak surveillance.
Alta DiagnoTech delivers cutting-edge IVD solutions for accurate and rapid tuberculosis (TB) diagnosis, empowering healthcare providers to combat TB more effectively worldwide. If you have related needs, please feel free to contact us for more information or product support.
References
| Cat.No | Product Name | Price |
|---|---|---|
| MK-QCY-0005 | Mycobacterium tuberculosis Drug Resistance Gene Test Kit (DNA Microarray Chip Method) | Add To Cart |
| MK-QCY-0006 | Mycobacterium tuberculosis Species Identification Kit (DNA Microarray) | Add To Cart |
| AS-QCY-0005 | Mycobacterium tuberculosis Drug Sensitization Kit | Add To Cart |
| BI-QCY-0016 | Mycobacterium tuberculosis Infection Test Kit | Add To Cart |
| NATR-HMM-0007 | Mycobacterium Tuberculosis Complex (MTB) Nucleic Acid Detection Reagent | Add To Cart |
| BT-QCY-0023 | Mycobacterium ulcerans Test Kit | Add To Cart |
| EK-YJL-0082 | Human Tuberculosis (TB) Antibody (IgM) ELISA Kit | Add To Cart |
| EK-YJL-0083 | Human Tuberculosis (TB) Antibody (IgG) ELISA Kit | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |