- Home
- Resource
- Disease Diagnosis
- Cancers
- Precision Diagnostics for Breast Cancer: The Evolving Role of IVD Biomarkers and Technologies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
With the advancement of in vitro diagnostics (IVD) technologies, breast cancer diagnostics are undergoing a revolution. This resource delves into key biomarkers, emerging technologies, and innovative products that are reshaping early detection, precise subtyping, and personalized treatment strategies. From liquid biopsies to artificial intelligence analysis, we explore how modern IVD innovations are addressing current challenges and shaping the future of precision breast cancer diagnostics.
Breast cancer is a malignant tumor originating in breast cells, primarily affecting women but also occurring in men. Key causes include genetic mutations (BRCA1/2), hormonal factors, and lifestyle influences. With approximately 2.3 million new cases annually worldwide (WHO), it represents 25% of all female cancers, creating significant healthcare and socioeconomic burdens. Early diagnosis through screening and advanced testing is crucial, as detecting breast cancer at stage I improves 5-year survival rates to over 90% compared to 30% for stage IV. This underscores the vital role of modern diagnostic technologies in saving lives and reducing treatment costs.
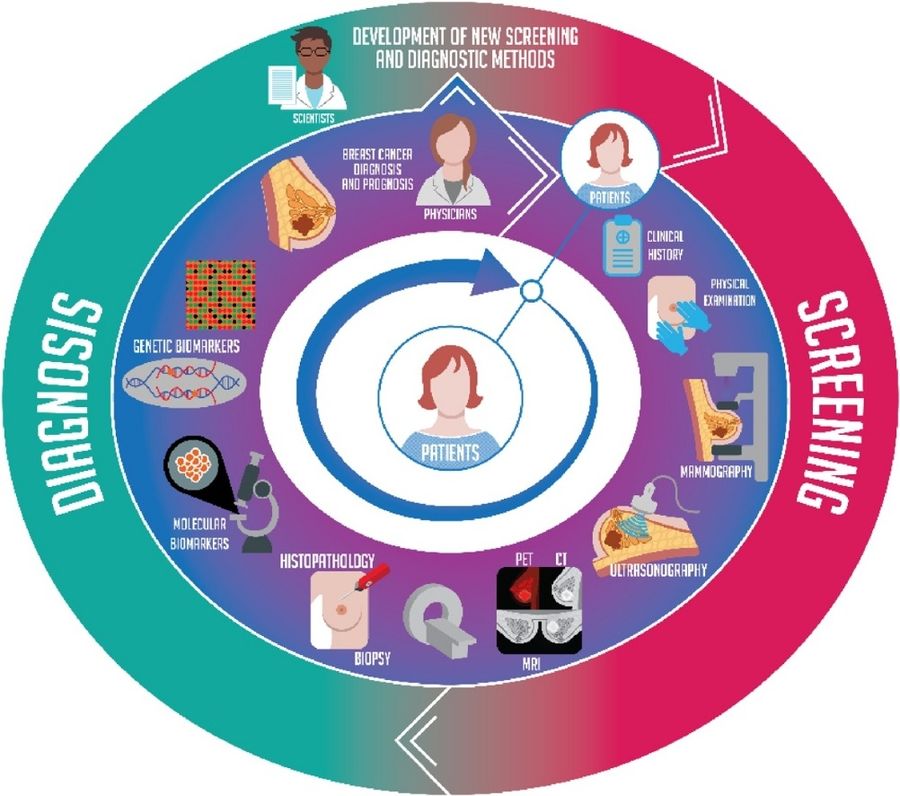 Fig.1 New screening and diagnostic methods for breast cancer. (Barba D, et al., 2021)
Fig.1 New screening and diagnostic methods for breast cancer. (Barba D, et al., 2021)
While breast cancer screening and diagnostics have improved significantly, critical limitations persist across three key areas: imaging accuracy, biomarker reliability, and tumor heterogeneity.
Mammography remains the frontline screening tool but suffers from 30-50% sensitivity loss in dense breast tissue and limited availability in resource-poor regions. While MRI and ultrasound improve detection, their high cost prevents widespread use, creating critical diagnostic gaps.
Traditional markers like CA 15-3 detect only 20-30% of early-stage cancers and frequently yield false positives. Their inability to differentiate subtypes or monitor dynamic tumor changes underscores the need for novel molecular biomarkers.
Breast cancer's molecular diversity (e.g., HER2+ vs. triple-negative) demands combined imaging, liquid biopsy, and genomic profiling for accurate diagnosis. Single-method approaches fail to address tumor evolution or guide personalized therapy effectively.
Biomarkers for breast cancer play a crucial role in modern in vitro diagnostics (IVD), facilitating non-invasive testing, molecular subtyping, and personalized treatment guidance. These biomarkers, measurable in blood, tissue, or other bodily fluids, overcome the limitations of imaging by providing quantifiable molecular information. They aid in early cancer detection, monitoring treatment response, and predicting the risk of recurrence, making them indispensable indicators in precision oncology.
| Biomarker Type | Examples | Role |
| Protein Biomarkers | HER2, ER/PR, CA 15-3, CEA | Subtyping, prognosis, and therapy selection (e.g., HER2-targeted treatments). |
| Genomic & Transcriptomic Biomarkers | BRCA1/2 mutations, Oncotype DX, PAM50 | Risk stratification, predicting recurrence, and guiding chemotherapy decisions. |
| Metabolic & Immunological Biomarkers | PD-L1, LDH, tumor-infiltrating lymphocytes (TILs) | Assessing immune response (immunotherapy suitability) and tumor metabolism. |
| Circulating Tumor Cells (CTCs) & Liquid Biopsy Markers | CTCs, ctDNA, exosomes | Non-invasive detection, monitoring metastasis, and tracking treatment resistance. |
Recent advances in in vitro diagnostics (IVD) are transforming breast cancer detection and management through innovative approaches that address current limitations in sensitivity, specificity, and clinical utility. Here we highlight key emerging technologies:
Advanced liquid biopsy technologies now enable ultra-sensitive detection of circulating tumor DNA (ctDNA) and cells (CTCs) from blood samples. These non-invasive tests provide real-time molecular profiling for early cancer detection, treatment monitoring, and identification of resistance mechanisms, significantly improving personalized cancer management.
Artificial intelligence (AI) is revolutionizing breast cancer diagnosis through deep learning algorithms that analyze complex imaging, pathology, and genomic data. These systems enhance diagnostic accuracy, predict treatment responses, and reduce interpretation variability, supporting more precise clinical decisions.
Miniaturized lab-on-a-chip platforms allow rapid, automated analysis of biomarkers at the patient's bedside. These portable systems enable CTC isolation, nucleic acid extraction, and molecular testing in resource-limited settings, making advanced diagnostics more accessible globally.

By integrating genomic, proteomic, metabolomic, and transcriptomic data, these comprehensive panels provide a holistic view of tumor biology. They improve diagnostic sensitivity, enable more accurate subtyping, and facilitate the development of targeted therapies tailored to individual patients' molecular profiles.
The integration of advanced biomarkers and cutting-edge IVD technologies enables the development of transformative diagnostic products that address critical gaps in breast cancer care. These solutions empower earlier detection, accurate subtyping, and personalized treatment strategies. Key product categories include:
Liquid Biopsy-Based Early Detection Kits
Point-of-Care Molecular Test Strips

AI-Driven Digital Pathology Systems
Multi-Omics Companion Diagnostic Platforms
Future of Breast Cancer IVD
The future of breast cancer in vitro diagnostics (IVD) lies in multimodal, AI-powered solutions that integrate liquid biopsy, multi-omics profiling, and point-of-care testing (POCT) to enable earlier detection, dynamic monitoring, and precision therapy. Advances in ultra-sensitive ctDNA detection (e.g., single-molecule sequencing), wearable microfluidic sensors, and standardized biomarker panels will shift diagnostics from reactive to proactive, patient-centric care.
Specializing in exploring comprehensive solutions for cancer in vitro diagnostics (IVD), Alta DiagnoTech offers a range of customized diagnostic products for breast cancer, including reagents, kits, equipment, and more. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| GD-QCY-0019 | Breast Cancer HER2 Mutation Test Kit | Add To Cart |
| GD-QCY-0020 | Breast Cancer HER2 Mutation CISH Test Kit | Add To Cart |
| GD-QCY-0021 | Breast Cancer PIK3CA Mutation RT-PCR Test Kit | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |