- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Norovirus Laboratory Detection: A Complete Guide to EIA, PCR, and Novel Assays
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Norovirus, the leading cause of acute viral gastroenteritis worldwide, demands accurate and timely diagnosis to control outbreaks and guide clinical management. This comprehensive resource examines the full spectrum of norovirus detection methods, comparing their clinical utility, limitations, and optimal use cases. Whether you're evaluating point-of-care options or laboratory-based confirmatory testing, understanding these diagnostic approaches is essential for effective norovirus management.
Norovirus, a highly contagious RNA virus of the Caliciviridae family, is the leading cause of acute gastroenteritis worldwide, responsible for an estimated 685 million annual cases. Known for its low infectious dose (as few as 18 viral particles) and environmental stability, the virus spreads rapidly through fecal-oral transmission, contaminated surfaces, or food/water. Outbreaks frequently occur in crowded settings (hospitals, cruise ships, schools) and present with sudden-onset vomiting, watery diarrhea, and abdominal cramps.
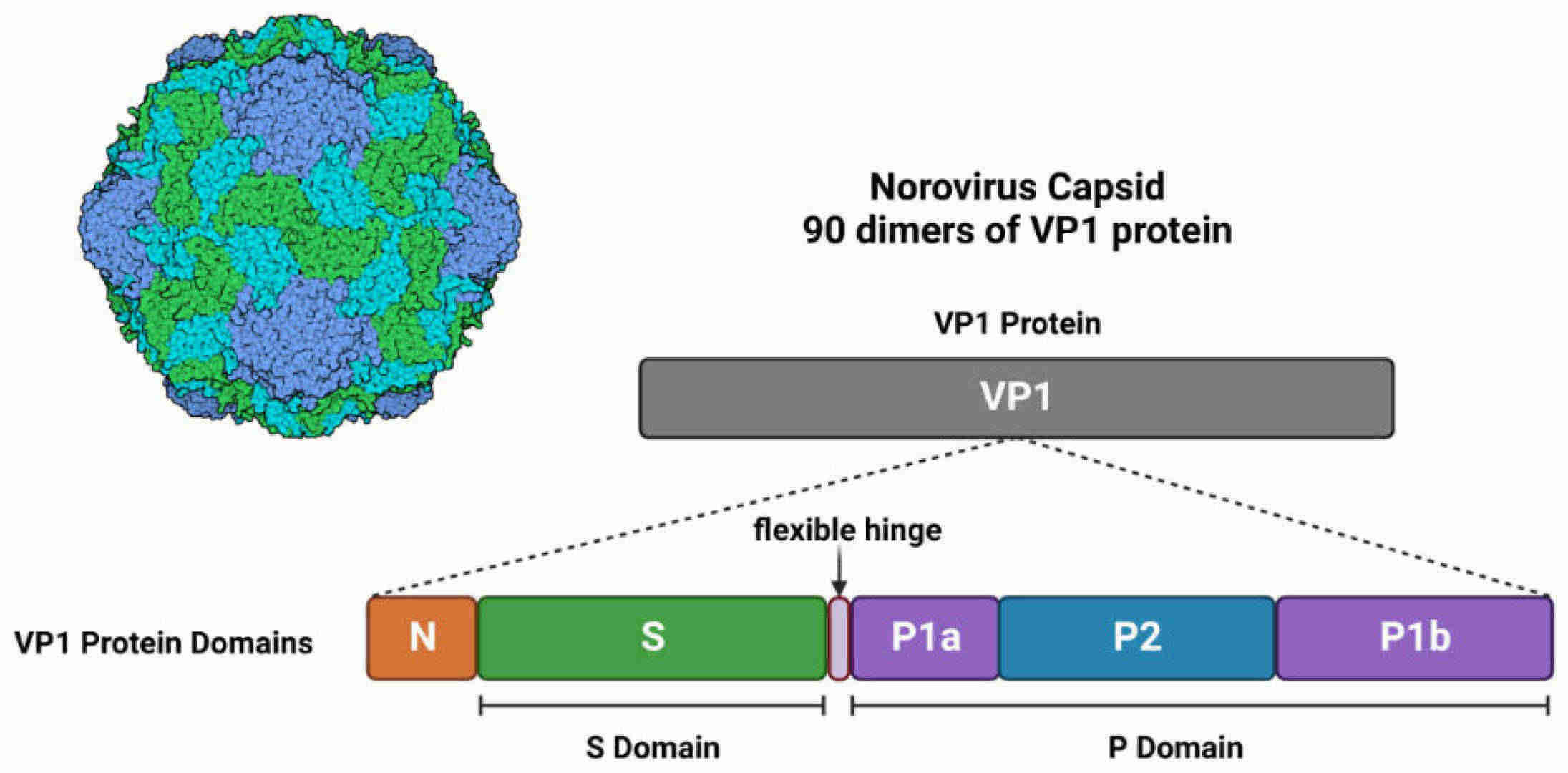 Fig.1 Structure of norovirus particles. (Winder N, et al., 2022)
Fig.1 Structure of norovirus particles. (Winder N, et al., 2022)
Diagnosis of norovirus infection plays a critical role in the identification and management of this highly contagious gastrointestinal illness. Timely and accurate diagnosis is essential for implementing appropriate infection control measures, preventing outbreaks, and developing targeted treatment strategies. As there is no specific antiviral treatment, diagnosis relies on laboratory detection of viral RNA or antigens, while prevention focuses on strict hygiene and disinfection. The high genetic diversity of the virus (particularly the GII.4 variant) and short-lived immunity pose ongoing challenges for diagnostics and vaccine development.
Accurate and timely diagnosis of norovirus infection is critical for clinical management and outbreak control. Given the virus's high genetic diversity and low infectious dose, laboratories rely on a combination of established and emerging techniques, each offering distinct advantages in sensitivity, speed, and applicability. Below, we explore three key diagnostic approaches: Enzyme Immunoassays (EIAs) for rapid screening, RT-qPCR as the gold standard for sensitivity and specificity, and Novel Assays pushing the boundaries of point-of-care and multiplex detection.

EIAs detect norovirus antigens (primarily VP1 capsid protein) in stool samples, providing results in 1–2 hours with minimal equipment. While cost-effective and user-friendly, their sensitivity (40–70%) is limited, particularly for non-GII.4 strains, making them more suitable for outbreak screening than individual diagnosis.

RT-qPCR targets conserved regions (e.g., RdRp or ORF1-ORF2 junction) with exceptional sensitivity (<10 viral copies) and strain-typing capability. Despite requiring specialized equipment and expertise, it remains indispensable for clinical diagnostics, outbreak investigations, and surveillance.
Emerging technologies like isothermal amplification (LAMP/RPA) offer portable, rapid alternatives to PCR, while multiplex panels simultaneously detect norovirus alongside other gastroenteritis pathogens. These innovations aim to bridge gaps in field-deployable, high-throughput testing.
| Parameter | EIA | RT-qPCR | LAMP |
| Target | VP1 capsid protein | Viral RNA (RdRp/ORF1-ORF2) | Viral RNA (specific regions) |
| Sensitivity | 40-70% | >95% (>10 copies/µL) | 85-95% (~50 copies/µL) |
| Specificity | Strain-dependent (GII.4 bias) | High (primer-specific) | High (6-8 primer sets) |
| Time to Result | 1-2 hours | 3-6 hours | 30-60 minutes |
| Equipment | Microplate reader | Thermal cycler, lab setup | Heating block/water bath |
| Best Use Case | Outbreak screening | Clinical diagnosis, surveillance | Near-patient molecular testing |
| Key Limitations | Low sensitivity for non-GII.4 | Equipment/reagent costs | Primer design complexity |
Norovirus diagnosis faces significant hurdles, including the virus's extreme genetic diversity (particularly emerging non-GII.4 strains), low infectious dose requiring ultra-sensitive assays, and lack of standardized rapid tests for point-of-care use. Current EIAs suffer from variable sensitivity (40–70%), while gold-standard RT-qPCR remains costly and infrastructure-dependent. Future solutions focus on field-deployable molecular tools, multiplex panels for simultaneous pathogen detection, and AI-assisted interpretation to enhance accuracy. Advances in biomarker discovery and affordable rapid diagnostics will be critical for outbreak control and vaccine efficacy monitoring.
Focused on providing comprehensive IVD solutions for norovirus infection, Alta DiagnoTech empowers norovirus laboratory testing by offering ultra-sensitive RT-qPCR kits and rapid antigen detection kits. If you have related needs, please feel free to contact us for more information or product support.
Reference
| Cat.No | Product Name | Price |
|---|---|---|
| CA-00147 | Norovirus rapid assay kit | Add To Cart |
| CA-00149 | Norovirus, rotavirus and adenovirus combo rapid assay kit | Add To Cart |
| CA-00151 | Norovirus, rotavirus, adenovirus and astrovirus combo rapid assay kit | Add To Cart |
| VT-QCY-0003 | Norovirus Type GI, GII RNA Nucleic Acid Test Kit (PCR-Fluorescent Probe Method) | Add To Cart |
| NATR-HMM-0045 | Bovine Norovirus (BoNoV) Nucleic Acid Detection Reagent (RT-PCR) | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |