Respiratory syncytial virus (RSV) remains a leading cause of severe respiratory infections in infants, elderly, and immunocompromised populations worldwide, demanding accurate and timely diagnosis to guide clinical management and public health interventions. This resource provides a thorough examination of current and emerging RSV diagnostic approaches, including rapid antigen tests (RADTs), molecular methods, and serological assays, while highlighting critical considerations for sample collection, method selection, and quality control.
Introduction to Respiratory Syncytial Virus Infection
Respiratory syncytial virus (RSV) is a highly contagious RNA virus and a leading cause of acute respiratory infections, particularly in infants, young children, and immunocompromised or elderly adults. Globally, RSV results in approximately 33 million lower respiratory tract infections annually, with severe cases leading to bronchiolitis, pneumonia, and 66,000 pediatric deaths, primarily in low-resource settings. The virus circulates seasonally, peaking in winter months in temperate regions, and presents with flu-like symptoms (cough, fever, wheezing), often requiring differential diagnosis from influenza or COVID-19. While most cases are mild, early detection is critical for high-risk groups to guide interventions.
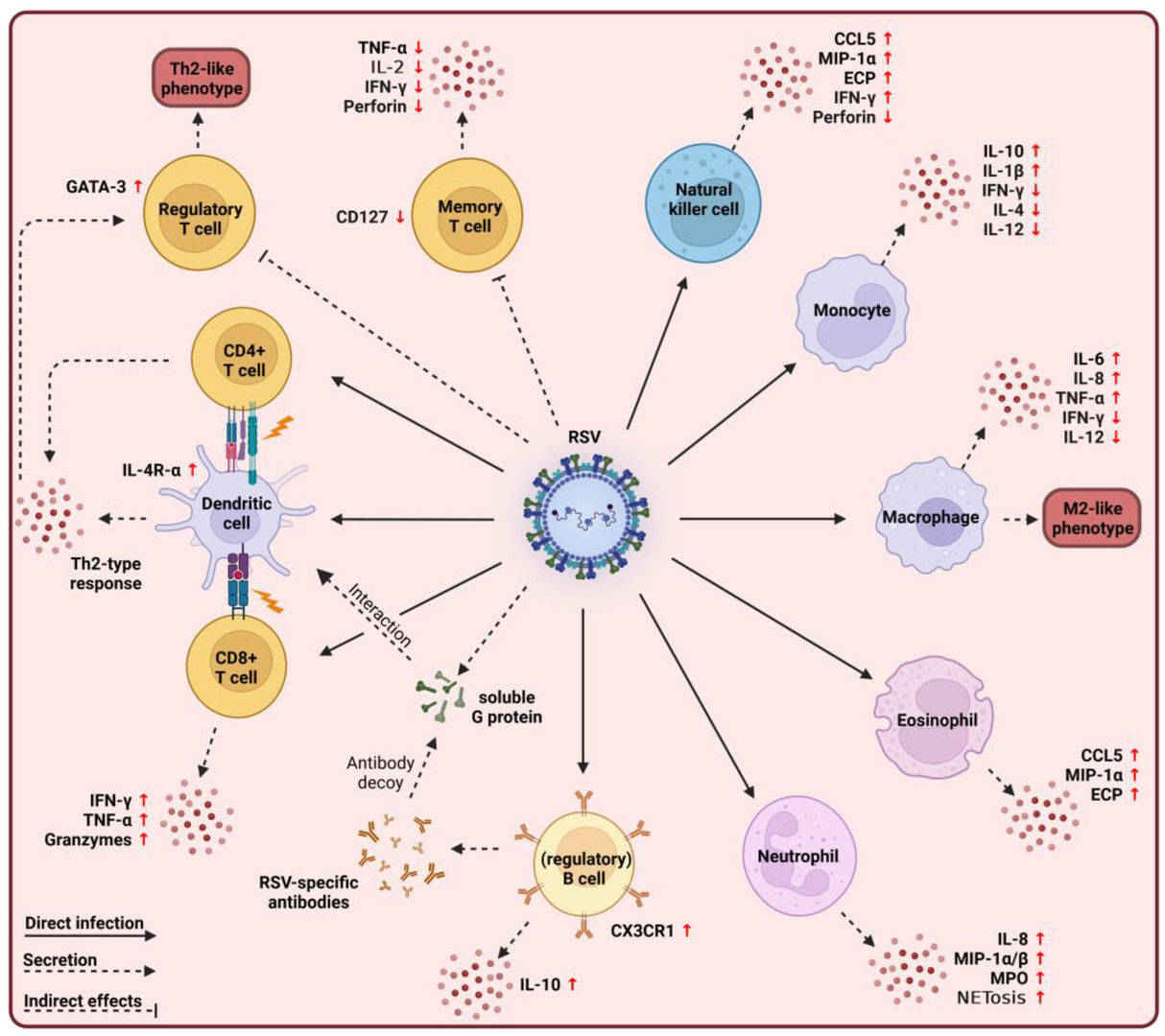 Fig.1 Overview of immunopathogenic responses mediated by innate and adaptive immune cells during respiratory syncytial virus (RSV) infection. (Agac A, et al., 2023)
Fig.1 Overview of immunopathogenic responses mediated by innate and adaptive immune cells during respiratory syncytial virus (RSV) infection. (Agac A, et al., 2023)
Laboratory Diagnostic Methods for Respiratory Syncytial Virus Infection
Accurate diagnosis of respiratory syncytial virus (RSV) infection relies on multiple laboratory techniques, each with distinct advantages depending on clinical needs, resource availability, and testing objectives. Key methods include viral culture for strain characterization, antigen detection for rapid screening, antibody detection for epidemiological studies, and nucleic acid testing (NAT) for high-sensitivity confirmation.
Viral Culture
Viral culture, traditionally the gold standard, isolates live RSV from nasopharyngeal specimens using cell lines (e.g., HEp-2, A549). While highly specific, its low sensitivity (60–70%) and prolonged turnaround time (3–7 days) limit clinical utility. Today, it is primarily reserved for research, strain typing, and antiviral susceptibility testing.
Antigen Detection
Rapid antigen detection tests (RADTs) identify RSV surface proteins (e.g., F protein) via lateral flow immunoassays. These tests provide results in 15–30 minutes and are widely used in point-of-care settings, but their sensitivity drops significantly in adults (50–80%) due to lower viral loads. RADTs are ideal for pediatric triage but require PCR confirmation in high-risk negative cases.
Antibody Detection
Serological assays (ELISA, immunofluorescence) detect RSV-specific IgM/IgG antibodies, primarily in serosurveillance and vaccine studies. Antibody responses appear late (1–2 weeks post-infection), making serology unsuitable for acute diagnosis. Cross-reactivity with other pneumoviruses and variability in immune responses further complicate interpretation.
Nucleic Acid Tests (NAT)
NAT, particularly real-time RT-PCR, is the most sensitive (>95%) and specific method for RSV detection, targeting conserved genes. It is the preferred choice for neonatal, elderly, and immunocompromised patients. Emerging NAT technologies, such as multiplex panels (simultaneous RSV/flu/COVID-19 detection) and isothermal amplification (LAMP), are advancing rapid, high-throughput testing.
Diagnostic Method Comparison for Respiratory Syncytial Virus Infection
| Parameter |
Viral Culture |
RADTs |
ELISA (Serology) |
RT-PCR |
| Target |
Live virus |
Viral antigens (F protein) |
RSV-specific antibodies (IgM/IgG) |
Viral RNA (N/F genes) |
| Sensitivity |
60–70% |
50–90% |
Variable (late antibodies) |
>95% |
| Specificity |
100% |
Moderate (cross-reactivity) |
Moderate (cross-virus) |
99–100% |
| Turnaround Time |
3–7 days |
15–30 minutes |
2–4 hours |
2–6 hours |
| Sample Type |
NP swab, aspirate |
NP swab |
Serum/plasma |
NP swab, BAL |
| Primary Use |
Research/strain typing |
Point-of-care screening |
Serosurveillance/vaccine studies |
Clinical confirmation |
| Advantages |
Gold-standard specificity |
Rapid, low-cost |
High-throughput |
High sensitivity/specificity |
| Disadvantages |
Slow, low sensitivity |
Low sensitivity in adults |
Late antibody rise |
Requires lab infrastructure |
Key Considerations for Respiratory Syncytial Virus Laboratory Testing
Accurate and reliable detection of respiratory syncytial virus (RSV) requires strict adherence to standardized protocols across all testing phases, from sample collection to result interpretation. Proper methodology ensures diagnostic accuracy, minimizes false negatives/positives, and supports timely clinical decisions, particularly for high-risk populations (infants, elderly, and immunocompromised patients).
- Sample Collection & Handling: Use flocked NP swabs stored in VTM at 4°C for optimal RSV detection, avoiding throat swabs and freeze-thaw cycles.
- Method-Specific Considerations: Select RADTs for rapid pediatric screening but confirm negatives with PCR, while reserving culture for research and serology for surveillance.
- Quality Control & Accuracy: Run controls with each batch and monitor for inhibitors to ensure reliable results.
- Biosafety & Laboratory Practices: Process infectious samples under BSL-2 conditions using appropriate PPE.
- Interpretation & Reporting: Report viral loads, subtype when relevant, and consider co-infections in result interpretation.
Future of Respiratory Syncytial Virus Infection Diagnosis
The future of respiratory syncytial virus (RSV) diagnosis is shifting toward ultra-rapid, highly sensitive, and accessible testing solutions, driven by innovations in point-of-care molecular assays, AI-powered diagnostic platforms, and non-invasive sampling (saliva/breath tests). Emerging multiplex panels will enable simultaneous detection of RSV alongside flu, COVID-19, and other respiratory pathogens, while wearable sensors and smartphone-integrated devices could revolutionize home monitoring for high-risk infants.
Alta DiagnoTech delivers comprehensive IVD solutions for respiratory syncytial virus (RSV) infection, offering high-performance PCR assay kits, rapid antigen test kits, and multiplex respiratory virus detection kits to enable accurate diagnosis across diverse healthcare settings. If you have related needs, please feel free to contact us for more information or product support.
Reference
- Agac A, Kolbe S M, Ludlow M, et al. Host responses to respiratory syncytial virus infection[J]. Viruses, 2023, 15(10): 1999.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Overview of immunopathogenic responses mediated by innate and adaptive immune cells during respiratory syncytial virus (RSV) infection. (Agac A, et al., 2023)
Fig.1 Overview of immunopathogenic responses mediated by innate and adaptive immune cells during respiratory syncytial virus (RSV) infection. (Agac A, et al., 2023)