Acquired immune deficiency syndrome (AIDS) remains a critical global health challenge, with early and accurate diagnosis being paramount to effective treatment and prevention. This resource provides a comprehensive overview of modern diagnostic approaches for HIV/AIDS, from cutting-edge screening technologies to confirmatory testing and emerging biomarker strategies.
Overview of Acquired Immune Deficiency Syndrome (AIDS)
Acquired immune deficiency syndrome (AIDS) is the most advanced stage of human immunodeficiency virus (HIV) infection, characterized by severe immune system damage (CD4 count <200 cells/μL) and life-threatening opportunistic infections or cancers. As a global health priority, AIDS diagnosis requires a multi-step approach: initial screening with 4th-generation antigen/antibody tests, confirmatory nucleic acid testing (e.g., PCR for HIV RNA), and ongoing monitoring through CD4 counts and viral load quantification.
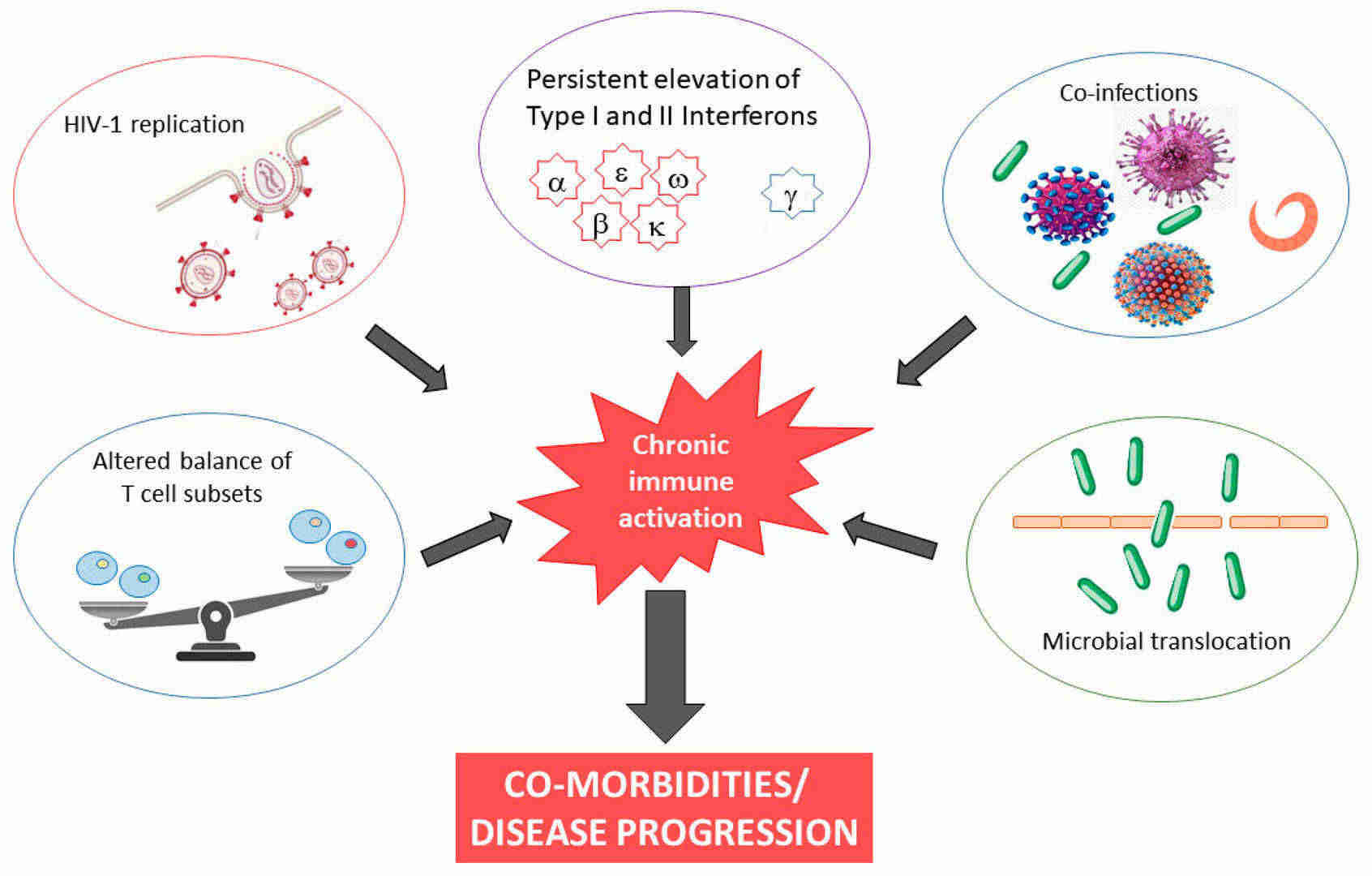 Fig.1 Causes and consequences of chronic immune activation in HIV-1 infection. (Mazzuti L, et al., 2023)
Fig.1 Causes and consequences of chronic immune activation in HIV-1 infection. (Mazzuti L, et al., 2023)
Screening Strategies for Acquired Immune Deficiency Syndrome (AIDS)
Effective screening for HIV/AIDS is critical for early detection and timely intervention to prevent disease progression and transmission. Modern screening strategies prioritize high sensitivity, rapid turnaround, and accessibility to meet diverse healthcare needs. Two key approaches, 4th-generation combination assays for laboratory-based testing and rapid point-of-care (POC) tests for decentralized settings, form the backbone of global HIV screening programs, each offering unique advantages for different clinical scenarios.
4th-Generation Combination Assays
4th-generation HIV tests simultaneously detect HIV-1/2 antibodies and p24 antigen, significantly reducing the diagnostic window period to 2–4 weeks post-exposure. These immunoassays achieve >99% sensitivity and are the gold standard for laboratory screening. Their ability to identify acute HIV infection (via p24 antigen) makes them indispensable for blood safety and early diagnosis, though they require trained personnel and lab infrastructure.
Rapid Point-of-Care (POC) Tests
Rapid POC tests provide results in 20–30 minutes using whole blood, oral fluid, or plasma, enabling testing in non-clinical settings (e.g., community outreach). While convenient, most POC tests are antibody-only, with lower sensitivity in acute infection (∼85–90% vs. 4th-gen assays). Newer multiplex POC combo tests now integrate p24 antigen detection, bridging this gap. Their simplicity supports high-risk population screening and self-testing initiatives.
Confirmatory & Differential Testing for Acquired Immune Deficiency Syndrome (AIDS)
Following reactive screening results, confirmatory testing is essential to definitively diagnose HIV infection and differentiate HIV-1 from HIV-2 or other rare variants. This critical step minimizes false positives and ensures accurate linkage to treatment. Two gold-standard approaches dominate this space: Western Blot/Line Immunoassay (LIA) for antibody specificity analysis, and nucleic acid testing (NAT) for direct viral detection, each serving distinct but complementary roles in the diagnostic algorithm.
Western Blot/Line Immunoassay (LIA)
Western Blot and Line Immunoassays (LIA) are antibody-based confirmatory tests that detect immune reactivity to specific HIV proteins (e.g., gp41, p24). Though historically the gold standard, these methods are being phased out in favor of NAT due to several limitations: they cannot detect acute HIV (as they rely on antibody development), require 1-2 days for results, and may yield indeterminate interpretations.
Nucleic Acid Testing (NAT)
Nucleic acid testing (NAT), primarily PCR-based, directly detects HIV RNA/DNA and has become the preferred confirmatory method. NAT offers unparalleled advantages: it identifies infection within 10-14 days post-exposure (far earlier than antibody tests), quantifies viral load for treatment monitoring, and differentiates HIV-1 from HIV-2. NAT is now recommended by WHO/CDC as the first-line confirmatory test following reactive 4th-generation assay results.
Biomarkers for Acquired Immune Deficiency Syndrome (AIDS) Diagnosis
Biomarkers play an increasingly critical role in the diagnosis, staging, and management of HIV/AIDS, offering insights beyond traditional viral load and CD4+ T-cell counts. These markers help identify disease progression, treatment efficacy, and immune recovery, enabling personalized care for patients.
- Virological markers directly measure HIV activity, including HIV RNA viral load (gold standard for detecting active infection and monitoring ART response), proviral DNA (assessing latent reservoirs for cure research), and p24 antigen (early acute-phase detection in 4th-gen assays). These markers are critical for diagnosing active infection, guiding treatment decisions, and evaluating viral suppression.
- Immunological markers assess immune dysfunction, notably CD4+ T-cell count (AIDS-defining if <200 cells/μL), CD4/CD8 ratio (predicting incomplete immune recovery), and soluble markers like sCD14 (indicating chronic inflammation). These help stage disease, predict complications, and monitor immune reconstitution post-ART.
- Host-derived biomarkers reflect systemic damage, including inflammatory cytokines (IL-6, CRP) linked to comorbidities, D-dimer (thrombosis risk), and miR-122 (liver injury). These aid in identifying non-AIDS-defining conditions (e.g., cardiovascular disease) and optimizing holistic patient management.
Future of Acquired Immune Deficiency Syndrome (AIDS) Diagnosis
The future of AIDS diagnosis is poised for transformation through point-of-care nucleic acid tests (POC NAT), ultrasensitive detection, and multi-omics profiling to enable earlier, more precise detection. Emerging technologies like smartphone-integrated rapid tests and AI-driven risk prediction algorithms aim to decentralize testing, while novel biomarkers may redefine treatment monitoring. These advances, coupled with self-testing innovations, strive to close diagnostic gaps in resource-limited settings and accelerate progress toward global HIV elimination targets.
Alta DiagnoTech provides comprehensive AIDS diagnostic solutions, including 4th-generation HIV Ag/Ab ELISA kits, rapid POC tests, and automated PCR systems for viral load quantification. If you have related needs, please feel free to contact us for more information or product support.
Reference
- Mazzuti L, Turriziani O, Mezzaroma I. The many faces of immune activation in HIV-1 infection: a multifactorial interconnection[J]. Biomedicines, 2023, 11(1): 159.
This article is for research use only. Do not use in any diagnostic or therapeutic application.



 Fig.1 Causes and consequences of chronic immune activation in HIV-1 infection. (Mazzuti L, et al., 2023)
Fig.1 Causes and consequences of chronic immune activation in HIV-1 infection. (Mazzuti L, et al., 2023)