- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Breaking Down Morbillivirus Detection: Molecular, Serological, and Point-of-Care Strategies
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Morbilliviruses like measles (MeV) and canine distemper (CDV) threaten both human and animal health, requiring precise diagnostic solutions. This resource examines modern detection methods, from gold-standard RT-PCR and sequencing to rapid point-of-care tests, that enable accurate diagnosis across clinical and field settings.
Morbilliviruses, a genus of highly contagious paramyxoviruses, include major pathogens such as measles virus (MeV) in humans, canine distemper virus (CDV) in animals, and peste des petits ruminants virus (PPRV) in livestock. These viruses share similar clinical manifestations (fever, rash, immunosuppression) and structural features but differ in host specificity and zoonotic potential. With measles alone causing >100,000 annual deaths globally and CDV threatening wildlife conservation, accurate diagnosis is critical for outbreak control, vaccination monitoring, and interspecies transmission prevention.
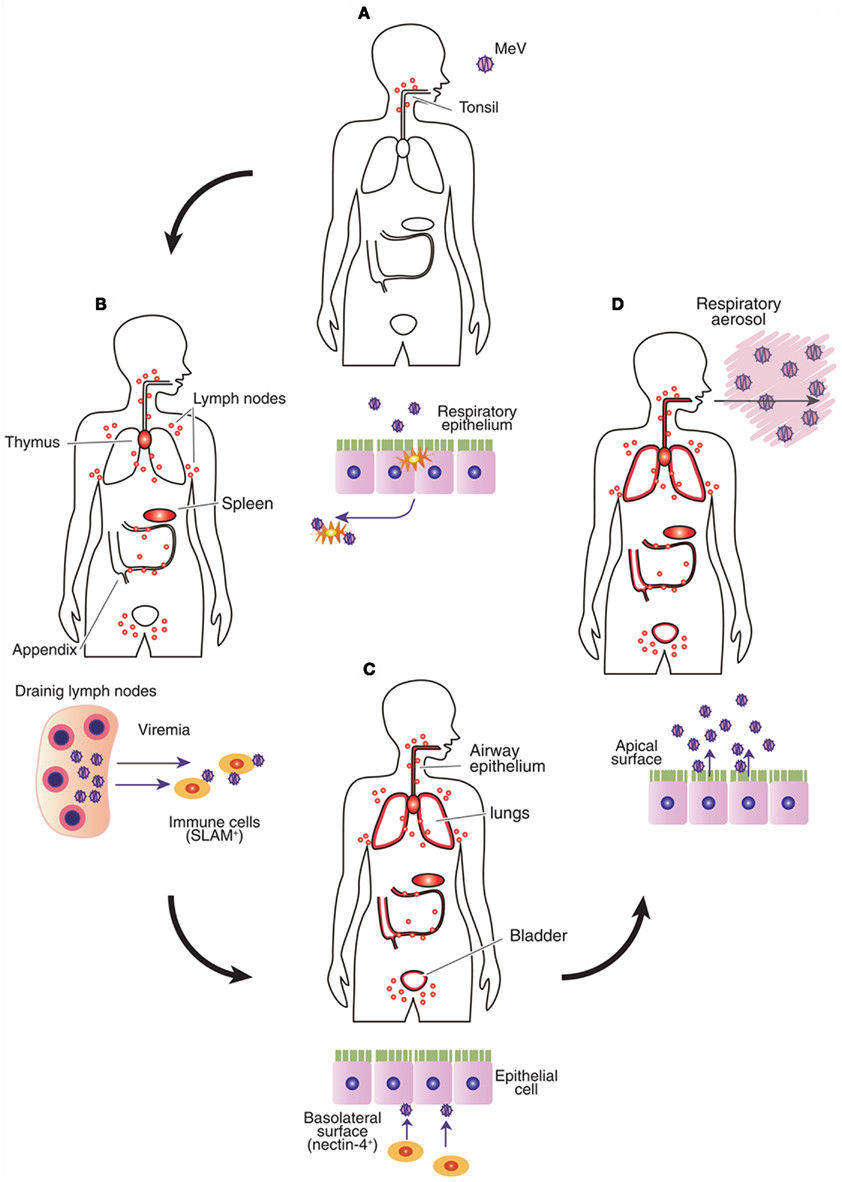 Fig.1 Major transmission mode of measles virus (MeV) in infected body. (Sato H, et al., 2012)
Fig.1 Major transmission mode of measles virus (MeV) in infected body. (Sato H, et al., 2012)
Molecular diagnostics are the gold standard for morbillivirus detection, offering high sensitivity and specificity to identify active infections, differentiate virus species (e.g., MeV vs. CDV), and track outbreaks. These methods target conserved viral genes (e.g., nucleocapsid N, hemagglutinin H) and are critical for early diagnosis, vaccine efficacy monitoring, and zoonotic surveillance. Below, we highlight two key approaches: RT-PCR for routine detection and sequencing for advanced characterization.
RT-PCR
RT-PCR is the frontline molecular method for acute morbillivirus diagnosis, amplifying viral RNA from clinical samples with >95% sensitivity. Real-time qPCR variants enable rapid (2-6 hour) detection and quantification, while multiplex assays simultaneously differentiate MeV, CDV, and PPRV by targeting conserved genes (N/H).
Sequencing
Sequencing (Sanger/NGS) analyzes genetic variation in the measles virus genome, providing strain-level resolution for outbreak tracking and vaccine surveillance. Portable platforms enable field-deployed sequencing, while NGS supports comprehensive surveillance for emerging strains in animal reservoirs.
Serological methods play a crucial role in diagnosing morbillivirus infections by detecting host immune responses rather than the virus itself. These tests are particularly valuable for identifying past exposure, confirming acute infections, and assessing vaccine-induced immunity.
IgM/IgG ELISA
IgM/IgG enzyme-linked immunosorbent assays (ELISAs) are widely used to diagnose morbillivirus infections by detecting virus-specific antibodies in serum. IgM antibodies indicate recent or acute infection, typically appearing within days of symptom onset, while IgG antibodies reflect past infection or vaccination and provide long-term immunity data.
Neutralization Tests
Neutralization tests, such as the plaque reduction neutralization test (PRNT), measure the ability of antibodies to neutralize live virus in vitro, providing a functional assessment of immunity. These tests are considered the gold standard for confirming protective immunity, particularly after vaccination. While highly specific, they require specialized biosafety labs and skilled personnel. Pseudovirus-based neutralization assays offer a safer alternative for high-throughput screening without handling live virus.
Point-of-care (POC) testing for morbilliviruses enables rapid, on-site detection—critical for outbreak containment in resource-limited settings and field applications. These decentralized tools prioritize speed, simplicity, and portability while maintaining diagnostic accuracy. Below, we highlight two transformative POC approaches: lateral flow assays (LFAs) for antibody/antigen detection and isothermal amplification for nucleic acid-based diagnosis.

LFAs provide ultra-rapid results (10–30 minutes) by detecting morbillivirus antigens (e.g., MeV nucleoprotein) or IgM/IgG antibodies in blood/serum. These disposable strips use gold nanoparticle-labeled antibodies for visual readouts, requiring no instrumentation. While LFAs are ideal for field use and low-resource clinics, their sensitivity is lower than lab-based tests, and they cannot differentiate virus species.
Isothermal methods (e.g., LAMP, RPA) amplify viral RNA at constant temperatures, enabling PCR-like sensitivity in <1 hour with portable devices. These systems are robust for field testing and eliminate the need for thermal cyclers. However, they require primer optimization to avoid cross-reactivity and may lack multiplexing capabilities compared to lab-based PCR.
This resource has comprehensively explored modern strategies for morbillivirus diagnosis, spanning gold-standard molecular methods (RT-PCR, sequencing), serological assays (ELISA, neutralization tests), and innovative point-of-care tools (LFAs, isothermal amplification). While RT-PCR remains the cornerstone for acute infection detection, serology provides critical immunity insights, and emerging POC technologies address field surveillance needs. Each of these technologies plays a crucial role in epidemic control and the integration of One Health.
Dedicated to advancing global health through innovative diagnostic solutions, Alta DiagnoTech offers a comprehensive IVD product portfolio for morbillivirus testing, ranging from high-precision laboratory tests to rapid point-of-care tests, providing accurate diagnoses for both human and veterinary medicine. If you have related needs, please feel free to contact us for more information or product support.
Reference
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |