- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Advancing Malaria Detection: From Traditional Lab Techniques to Modern Molecular Diagnostics
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Malaria remains a global health challenge that requires accurate and accessible diagnostic solutions to guide treatment and control efforts. This resource provides a comprehensive overview of traditional and molecular diagnostic methods, from microscopy and rapid tests to advanced PCR and isothermal amplification, and compares these methods to guide clinical, laboratory, and public health agencies in selecting the optimal test, thereby supporting informed decision-making for effective malaria management.
Malaria is a life-threatening infectious disease caused by Plasmodium parasites, primarily transmitted through the bite of infected Anopheles mosquitoes. It remains a major global health challenge, with an estimated 247 million cases and 619,000 deaths annually, predominantly in sub-Saharan Africa. The disease manifests with symptoms such as fever, chills, anemia, and organ failure in severe cases. Five Plasmodium species (P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi) infect humans, with P. falciparum being the most lethal.
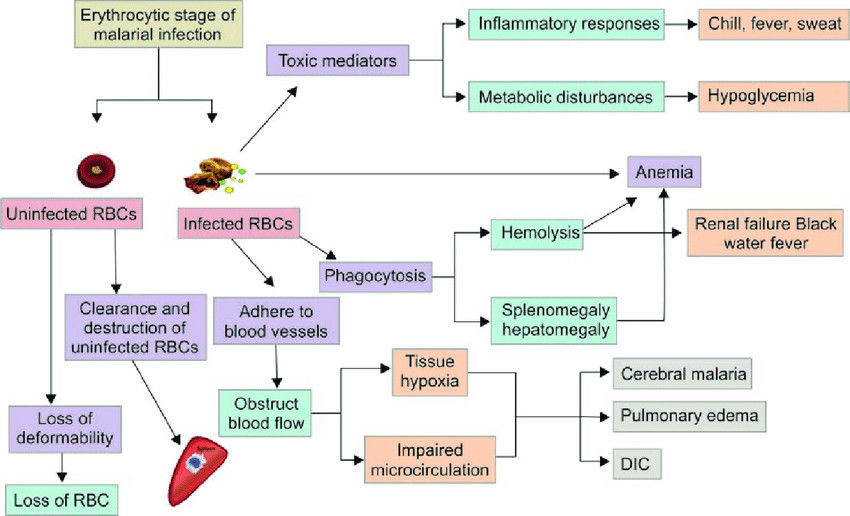 Fig.1 Pathogenesis of malarial infection. (Saxena R, et al., 2017)
Fig.1 Pathogenesis of malarial infection. (Saxena R, et al., 2017)
Accurate and timely diagnosis of malaria is critical for effective treatment, reducing transmission, and preventing severe outcomes. Rapid detection enables targeted antimalarial therapy, curbing unnecessary drug use and slowing resistance. Current diagnostic methods include microscopy, rapid diagnostic tests (RDTs), and molecular techniques like PCR. Despite advancements, challenges persist, particularly in resource-limited settings, such as variable test accuracy, lack of infrastructure, and emerging parasite mutations.
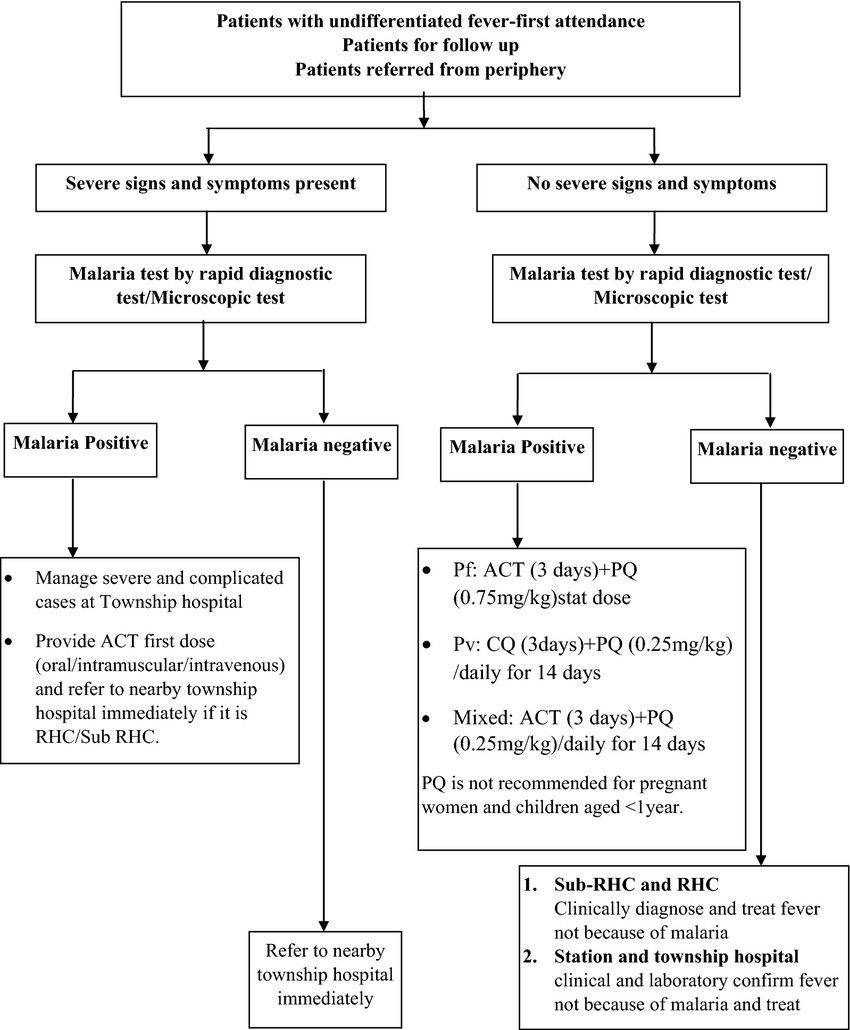 Fig.2 The algorithm of malaria screening, diagnosis and treatment services provided by basic health staffs. (Linn N Y Y, et al., 2018)
Fig.2 The algorithm of malaria screening, diagnosis and treatment services provided by basic health staffs. (Linn N Y Y, et al., 2018)
Malaria diagnosis has long relied on well-established laboratory methods that balance accuracy, accessibility, and practicality in diverse clinical settings. Two cornerstone techniques, microscopy and rapid diagnostic tests (RDTs), form the backbone of malaria detection worldwide, each offering distinct advantages and limitations. While newer molecular methods are emerging, these traditional approaches remain indispensable, particularly in resource-limited regions where malaria burden is highest.

Microscopic examination of Giemsa-stained blood smears remains the gold standard for malaria diagnosis, offering unparalleled specificity in species identification and parasite quantification. This technique allows visualization of Plasmodium parasites at different life stages, enabling clinicians to determine infection severity and monitor treatment response.

RDTs revolutionized malaria diagnosis by providing rapid, equipment-free detection through immunochromatographic assays targeting parasite antigens like PfHRP-2 and pLDH. These tests deliver results in 15-20 minutes, making them ideal for remote settings lacking laboratory infrastructure.
The field of malaria diagnostics has been transformed by molecular techniques that offer unprecedented sensitivity and specificity in parasite detection. These advanced methods overcome many limitations of traditional diagnostics by directly targeting Plasmodium DNA/RNA, enabling accurate identification even in low-parasitemia and mixed infections. As the need for precise surveillance and drug resistance monitoring grows, molecular diagnostics are becoming increasingly vital in both clinical and research settings, though their implementation requires careful consideration of technical and infrastructural requirements.
RCR-based techniques, including conventional, nested, and real-time PCR, represent the cornerstone of molecular malaria diagnostics. These methods amplify specific Plasmodium DNA sequences, achieving exceptional sensitivity (detecting 1-5 parasites/μL) and enabling species differentiation and quantification. Real-time PCR offers additional advantages with faster turnaround times (2-4 hours) and the ability to monitor amplification in real-time.
Isothermal amplification techniques like Loop-Mediated Isothermal Amplification (LAMP) and Nucleic Acid Sequence-Based Amplification (NASBA) provide molecular-level accuracy without the need for thermal cycling equipment. LAMP, for instance, can amplify DNA at a constant temperature (60-65°C) using simple heating blocks or water baths, yielding results in 30-60 minutes with visual readouts. These methods bridge the gap between basic RDTs and complex PCR systems, offering field-deployable molecular testing.
| Parameter | Microscopy | RDTs | PCR | LAMP |
| Basis of Detection | Visual parasite identification | Antigen detection | DNA amplification | DNA/RNA amplification |
| Sensitivity | 50-100 parasites/μL | 100-200 parasites/μL | 1-5 parasites/μL | 5-10 parasites/μL |
| Specificity | High (with expertise) | Moderate (varies by antigen) | Very high | High |
| Turnaround Time | 30-60 minutes | 15-20 minutes | 2-6 hours | 30-60 minutes |
| Equipment Needed | Microscope, reagents | None | Thermal cycler, lab equipment | Heating block/water bath |
| Quantification | Yes (parasite count) | No | Yes (real-time PCR) | Semi-quantitative |
| Key Limitations | Expertise-dependent, time-consuming | PfHRP-2 deletions, heat sensitivity | High cost, infrastructure needs | Primer design complexity, contamination risk |
Alta DiagnoTech delivers end-to-end IVD solutions for malaria diagnosis, offering premium test kits and cutting-edge diagnostic equipment tailored to diverse clinical settings and operational requirements. If you have related needs, please feel free to contact us for more information or product support.
References
| Cat.No | Product Name | Price |
|---|---|---|
| EA-00166 | Malaria rapid test kit | Add To Cart |
| EA-00167 | Malaria rapid assay kit | Add To Cart |
| EA-00168 | Malaria assay kit | Add To Cart |
| EA-00169 | Malaria test kit | Add To Cart |
| EA-00170 | Malaria rapid assay kit, 25 T | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |