- Home
- Resource
- Disease Diagnosis
- Infectious Diseases
- Dengue Fever Diagnosis Decoded: Standardized Testing Pathways for Accurate Detection
- Home
- IVD
- By Technology Types
- By Diseases Types
- By Product Types
- Research
- Resource
- Distributors
- Company
Dengue fever presents significant diagnostic challenges due to its dynamic clinical progression and symptom overlap with other arboviral diseases. This resource provides a standardized framework for dengue testing, covering optimal methodologies (RT-PCR, NS1 antigen, and IgM/IgG serology), phase-specific testing windows, and interpretation guidelines to ensure accurate diagnosis across primary and secondary infections.
Dengue fever is a mosquito-borne viral illness caused by the dengue virus (DENV, serotypes 1–4), primarily transmitted by Aedes aegypti mosquitoes. It ranges from mild febrile symptoms to severe, life-threatening manifestations like dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS), especially in secondary infections. With 390 million annual infections globally, dengue poses a significant public health burden in tropical and subtropical regions.
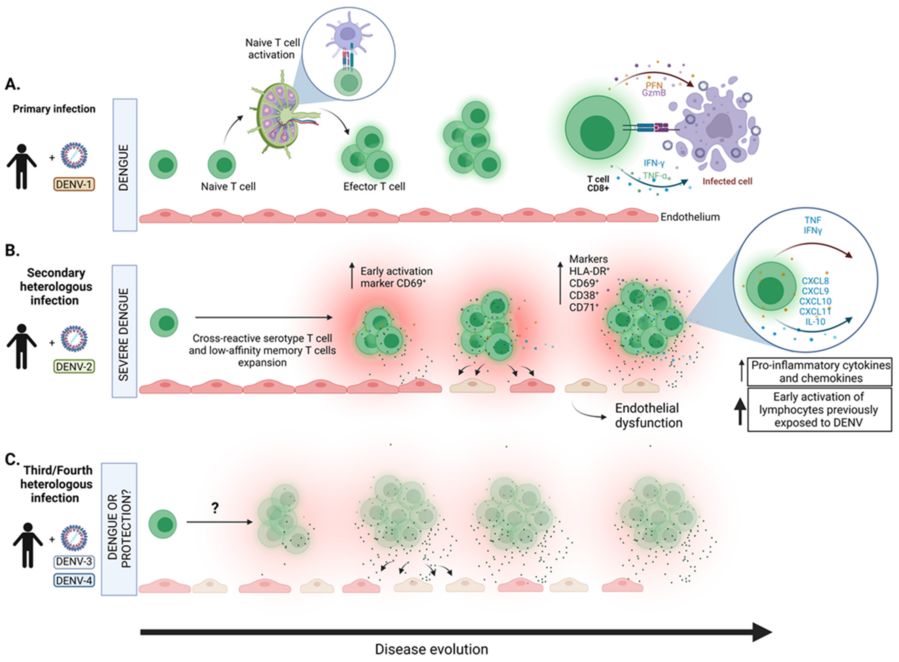 Fig.1 T cell responses during dengue virus infection. (Khanam A, et al., 2022)
Fig.1 T cell responses during dengue virus infection. (Khanam A, et al., 2022)
Accurate and timely diagnosis of dengue fever is essential to prevent serious complications, guide appropriate treatment, and support public health surveillance. The diagnosis of dengue fever remains a major challenge because its early symptoms, such as high fever, headache, and rash, are non-specific and overlap with other arboviruses (such as Zika and Chikungunya). Other challenges include changes in virus detection capabilities with the stage of disease and serological cross-reactivity. Therefore, standardized, context-aware testing strategies are needed to ensure accurate detection and optimal clinical management.
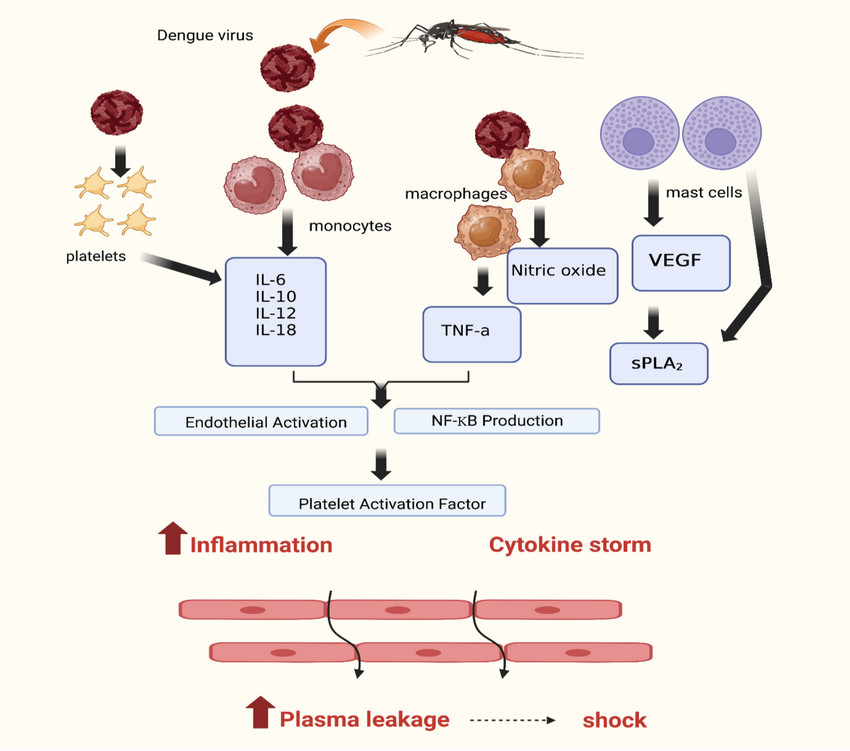 Fig.2 Pathophysiology of dengue-induced inflammation and shock. (Rehman B, et al., 2024)
Fig.2 Pathophysiology of dengue-induced inflammation and shock. (Rehman B, et al., 2024)
Dengue fever diagnosis requires phase-specific testing strategies to address the virus's dynamic presentation.
During the first 5 days post-symptom onset (early infection), direct detection of the virus through molecular (RT-PCR) or antigen (NS1) tests is critical, as viremia peaks in this window.
RT-PCR (Gold Standard)
As the most reliable method for early diagnosis, RT-PCR detects dengue viral RNA (targeting NS1/E/PrM genes) with >95% sensitivity during the first 5 days of symptoms. It identifies specific serotypes (DENV-1 to -4), crucial for outbreak tracking and clinical management.
NS1 Antigen Tests
NS1 rapid tests offer point-of-care detection of dengue viral protein via lateral flow or ELISA, delivering results in 15–30 minutes. These tests have high specificity (>90%) but variable sensitivity (70-90%), making them ideal for resource-limited settings and outbreak screening.
From day 4–7 onward (convalescent phase), serological tests (IgM/IgG) become essential to identify antibody responses and differentiate primary from secondary infections.
IgM ELISA detects dengue-specific IgM antibodies, typically appearing 4-7 days post-symptom onset and peaking at 2 weeks. With 80-95% sensitivity after day 7, it is ideal for confirming recent infections. However, cross-reactivity with other flaviviruses (e.g., Zika) requires careful interpretation, and paired testing (acute/convalescent samples) improves accuracy in borderline cases.

IgG ELISA measures dengue-specific IgG antibodies, which rise earlier and higher in secondary infections (indicating severe dengue risk). While useful for seroprevalence studies, standalone IgG results are less diagnostic—interpretation requires comparison with IgM levels and clinical history to distinguish primary (IgM-dominant) from secondary (IgG-dominant) infections.
| Method | Target | Optimal Timing | Sensitivity | Advantages | Limitations |
| RT-PCR | Viral RNA (NS1/E/PrM) | Days 1–5 of symptoms | >95% | Gold standard, serotype identification | Lab-dependent, short detection window |
| NS1 Antigen | Viral NS1 protein | Days 1–5 of symptoms | ~70-90% | Rapid POC use, low cost | Lower sensitivity in secondary infections |
| IgM ELISA | Anti-dengue IgM | Day 4–7 onward | ~80-95% | Confirms recent infection | Cross-reactivity |
| IgG ELISA | Anti-dengue IgG | Day 7–10 onward | High in late phase | Differentiates primary/secondary infections | Requires paired samples for accuracy |
The evolution of dengue diagnostics is advancing toward multiplex assays, portable high-precision testing, and AI-driven platforms. Innovations aim to address current gaps, including variant resilience, improved POC sensitivity, and integration with digital surveillance systems, while reducing costs for resource-limited settings. Future strategies will prioritize universal diagnostic protocols, non-invasive sampling (e.g., saliva/breathalyzers), and real-time data sharing to strengthen global outbreak response.
Focusing on providing comprehensive in vitro diagnostic (IVD) solutions for dengue fever, Alta DiagnoTech carefully prepares high-accuracy PCR tests, rapid antigen tests, and reliable serology kits to support precise detection and effective clinical management. If you have related needs, please feel free to contact us for more information or product support.
References
| Cat.No | Product Name | Price |
|---|---|---|
| CA-00155 | Dengue IgG/IgM rapid assay kit | Add To Cart |
| CA-00156 | Dengue NS1 rapid assay kit | Add To Cart |
| CA-00157 | Dengue IgG/IgM and NS1 combo rapid assay kit | Add To Cart |
| VI-QCY-0003 | Dengue Virus Infection PCR Test Kit | Add To Cart |
This article is for research use only. Do not use in any diagnostic or therapeutic application.
|
There is no product in your cart. |